By Ritter Medical…
Ritter Medical warns of rinse aid risks
Schwabmünchen, Germany: – Ritter Medical, leading manufacturer of liquid handling and robotic consumables products, has warned that the use of rinse aids in automatic decontamination systems is potentially unsafe when dealing with advanced plastic storage containers. The warning is incorporated in the updated user manual for Ritter’s widely used polySteribox® reusable sterilization, transport and storage container system, which states:
“The use of drying aids, in particular neodisher MediKlar from the company Chemische Fabrik Dr. Weigert, is strongly discouraged!”
The manual recommends rinsing in deionized water as the only safe procedure, together with strict observance of the temperature guidelines laid down in the KRINKO, BfArM and RKI recommendations for thermal and chemical disinfection.
Industry wide issue
“We are concerned that the surfactants, polycarboxylates or preservation agents like quaternium 15 and methylisothiazolinone used in rinse/drying aids like neodisher MediKlar can attack and degrade the plastics used in the polySteribox, causing brittleness and stress cracks that can compromise airtightness and sealing,” explained Ritter Key Account Manager Andreas Rauch.
“This is an issue of which the whole healthcare and life sciences industry should be aware,” said Rauch.
“The previous user instructions were adequate for purpose but we felt they could be even better and more comprehensive,” explained Ritter Key Account Manager Andreas Rauch.
“In particular we wanted to address the different sterilization technologies as well as the current standards such as EN 285, MDD 93/42/EEC and EN ISO 13485,” he said.
The new manual is available in German, English, French, Italian and Spanish language versions, available as .pdf printable portable document downloads by contacting the Ritter sales staff.
“The various language versions reflect a truly global user base for this very versatile and popular product. A Turkish version is in latter stages of proofing and graphic design and should be completed very soon,” Rauch added.
Advanced plastics
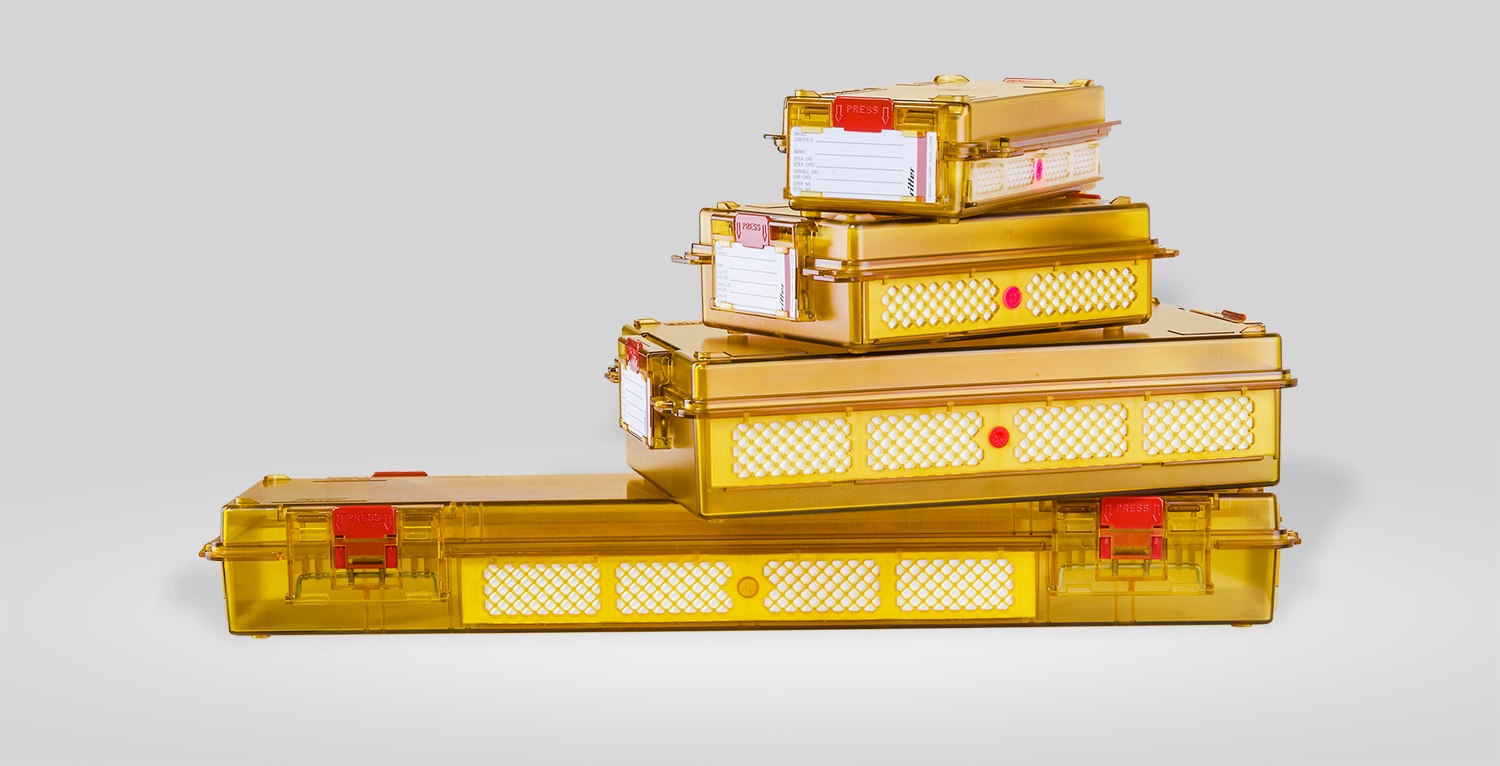
The polySteribox® containers are available in a range of sizes and shapes and are constructed from technically advanced plastics formulated for stability and high temperatures to ease sterilization. The boxes are designed to be securely locked, sealed and labeled, hygienically handled and with a stackable shape for optimum fitment into sterilization cabinets.
The manual covers locking and unlocking procedures, cleaning, use of tamper-proof seals, filter changes, storage and sterilization procedures, as well as storage durations extending up to five years.
The polySteribox is part of the Ritter Medical advanced plastics range featured in the company’s updated Clinical Products brochure, downloadable online from Ritter website.
Ritter polySteribox® in detail
Ritter’s polySteribox® is a range of reusable sterilization, transport and storage containers that are suitable for vacuum steam autoclave sterilization at 121 °C and 134 °C (up to 3 bar pressure) or for formaldehyde, ethylene oxide sterilization at 65 °C. The containers are also compatible with STERRAD® gas-plasma sterilization.
The combination of stable plastics, seals and filters form effective built-in barriers against germ and bacteria contamination following sterilization.
Simple and easy handling of sterile products is aided by the use of transparent materials, built-in label holders, sterilization seal system, positioning mats, simple and direct access to sterilized contents in polySteribox®, along with a stackable form that allows Individual or standardized sets to be combined.
Compared with metal containers, the polySteribox provides easier visibility of contents, no need to change silicone seals, and zero risk of heat deformation allowing sealing to be breached.
Specifications:
| Material: | Extremely stable special kind of plastic, temperature-resistant up to 150 °C |
| Shelf life: | Up to 5 years (with 250 sterilizations/uses per year or 1250 cycles in total) |
| Applications: | Hospital A&E departments, operating theatres, outpatients, neurosurgery, minor surgery, catheter sets, ENT wards, ophthalmic wards, etc. Practice clinics in General Practice, ENT, dental, veterinary, podiatrist; etc. |
| Available sizes: | SH, M, L, XL |
About Ritter Medical
Ritter, founded in Bavaria in 1965 is an independent family-owned company that has become a world-class manufacturer of high quality plastics and “made in Germany” sterile laboratory equipment, high precision dispensers, tips and other disposables.
Scientists and medical professionals in more than 40 countries rely on the company’s products. Ritter’s cartridge and medical products are particularly important in dialysis, dispensing and biotechnology applications. Many hospitals and laboratories also use Ritter disposables, dispensers and liquid handling systems.
Ritter’s comprehensive ranges of sterile assay and sample handling containers, robotic tips and other specialized equipment make its brand highly respected in research and biotech laboratories.
At its 25,500m2 production facility, Ritter’s 300 employees develop and produce high-quality plastic products for world markets, certified under ISO 9001 and ISO 13485. Ritter Medical only uses certified virgin plastics as raw material, with manufacturing processes that comply with ISO 50001 sustainability and performance standards.
Contact
Name: Andreas Rauch, Key Account Manager, Ritter GmbH | Medical
Tel: +49 823 250 037 20 / +49 171 831 556 7 (mob)
Email: [email protected]
Resources
Click on Ritter Clinical Products to download brochure.
For German version click here.
For English version click here.
For French version click here.
For Italian version click here.
For Spanish version click here.