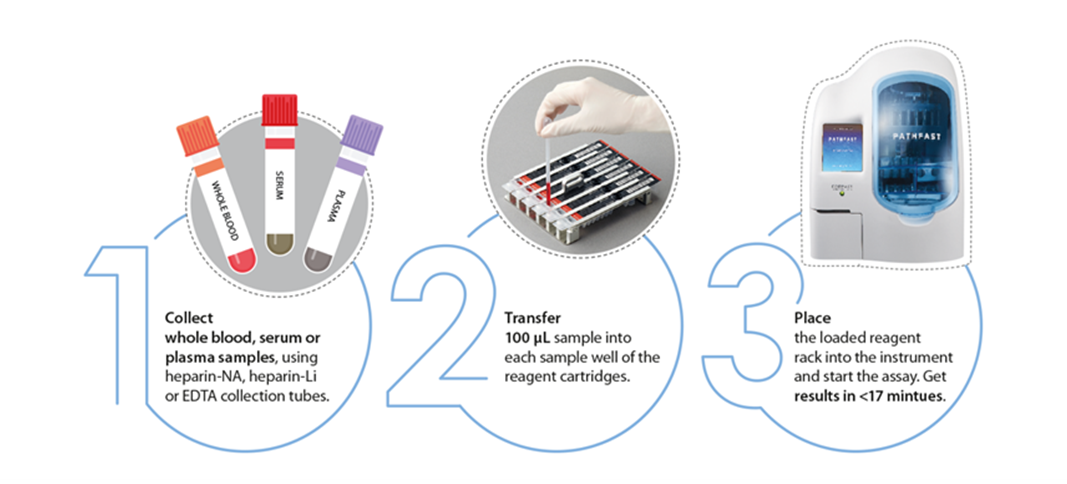
By PHC Europe…
PHCbi TB LAM Ag Test for PATHFAST® Immunoanalyzer
PHCbi has added a fast and easy diagnostic test for Tuberculosis (TB) to the range of plug-in cartridges for its benchtop immunoassay analyzer PATHFAST®.
The PATHFAST® TB LAM Ag Test cartridge3 is fully automatic bioassay that combines progressive chemiluminescence technology with PHCbi’s patented Magtration® technology to provide an in vitro testing solution that is highly sensitive and requires minimal containment.
PATHFAST™ TB LAM Ag Test Features
PATHFAST® TB LAM Ag test utilizes advanced technology to provide quantitative measurement of lipoarabinomannan (LAM) in human sputum. This can be used in laboratories, hospitals or clinics to provide a plug and play test for presence of TB that can return a result within 17 minutes.
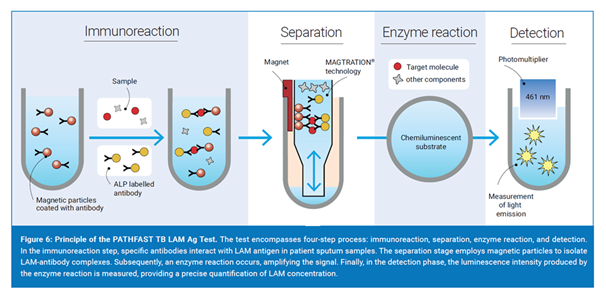
LAM is a 17.5 kDa glycolipid present in the mycobacterial cell wall and that is released during active TB infection. LAM can be detected in the sputum of TB patients with concentrations that closely correlate with smear microscopy scores and time to detect of a culture.
The LAM Ag test uses special antibodies that create a visible signal to indicate TB treatment success. The PATHFAST® analyzer employs a technology involving magnetic particles in a pipette tip. During the test, the sample interacts with specific antibodies and magnetic particles. After removing unwanted substances, a chemiluminescent substance (CDP-star) is added. The brightness produced during the test is used to calculate the LAM concentration in the sample.
All required components for performing the testing are packed in one reagent cartridge that can be slotted into the PATHFAST® immunoanalyzer.
PATHFAST® TB LAM Ag Advantages
The PATHFAST® TB LAM Ag test provides a highly precise, fast and compact chemiluminescence immunoassay analysis solution with many practical benefits in TB treatment monitoring:
- Minimal containment requirements with no need for high-level BSL3 or similar biosafety containment.
- Easy to use with straightforward operation and streamlined workflow requiring minimal training.
- Quantitative precision with highly correlated quantitative results that allow more accurate monitoring of TB treatment efficacy.
- High sensitivity in detecting low LAM concentrations, enabling treatment monitoring in patients with low TB bacterial loads.
- Swift result turnaround time of less than an hour expedites clinical decision-making.
- Data-driven decisions: Its quantitative nature enables tracking of LAM concentration changes over time.
- Cost-effective: Considering its speed and accuracy, it offers a cost-effective solution for TB management.
- Effective as Point-of-Care test with potential to bring TB diagnosis and treatment monitoring closer to patients in primary care facilities and clinics.
- All in one solution: Once sample preparation is complete, no further materials or equipment needed.
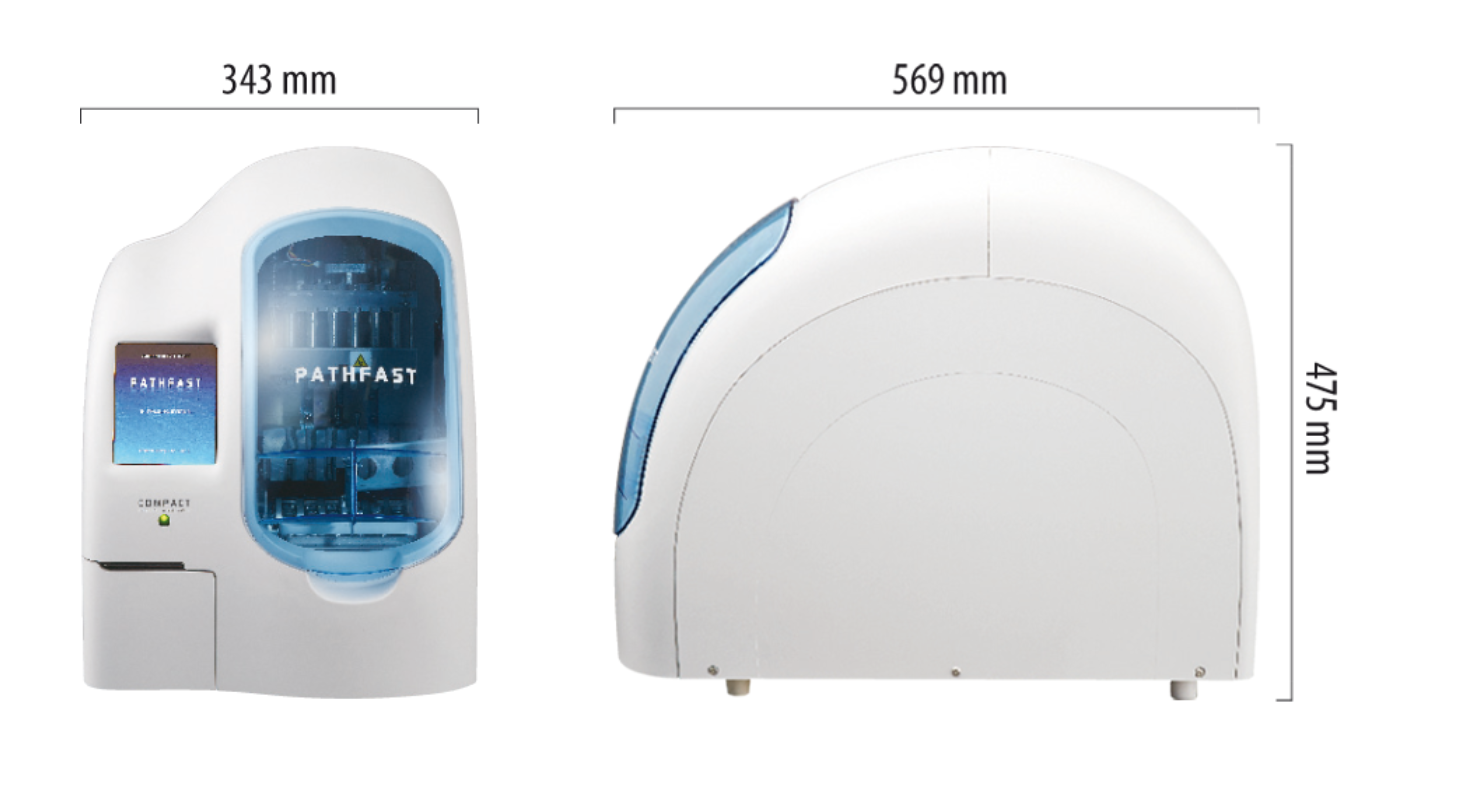
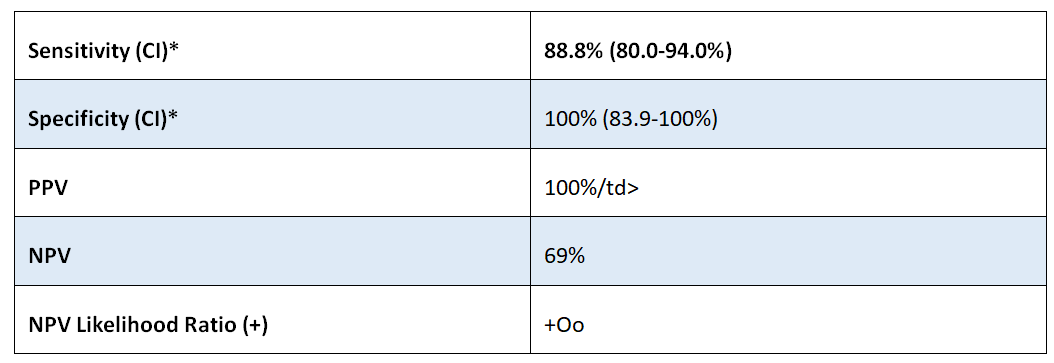
PATHFAST® TB LAM Ag Specifications
Resources
Click on TB LAM Ag Product Flyer to download brochure.
Click on PHCbi PATHFAST® compact immunoanalyzer for system information.
Click on PATHFAST® Emergency Markers to explore range of reagent strips available.
Click on PATHFAST® Sepsis Markers to explore range of emergency reagent strips available.
Click on PHCbi PATHFAST® Customer Testimonials to watch YouTube video of PATHFAST® in action.