By Molnár-Institute for…
Molnár-Institute releases DryLab® Empower Automation module
Berlin: – The Molnár-Institute for Applied Chromatography has rolled out the Empower Automation module as a significant expansion of its proprietary DryLab®4 chromatography modeling software that allows greater distributed networked collaboration and remote working.
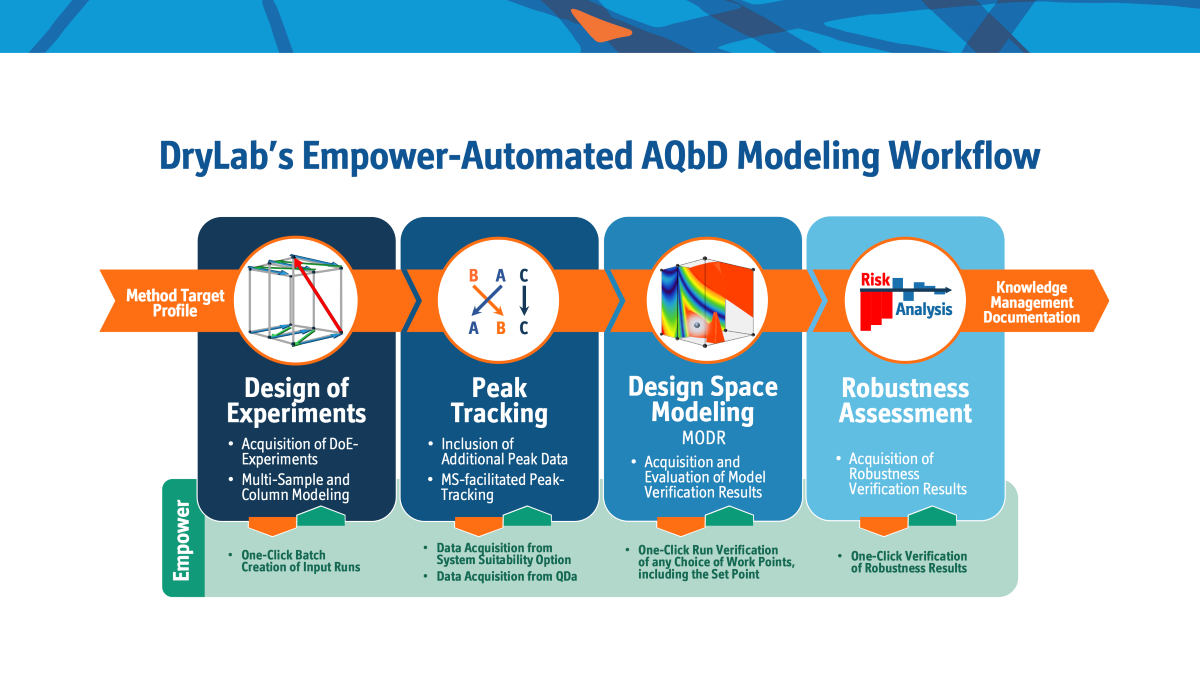
Co-developed with Waters, the Empower 3 Automation module introduces automated working on three levels of the DryLab-AQbD-workflow by creating, performing and retrieving input runs, automating model verification and robustness validation.
Complete method development
The new, completely automated and digitalized connection of DryLab® and Empower equips the chromatographer with an easy to use modeling platform. It allows for science-based, flexible decisions in method development, mitigated risk, improved analytical throughput and the most complete approach to successful method development.
The Empower module runs generated method sets in the most economic and ecological order, taking into account subsequent equilibration steps. DryLab® then accesses the Empower data-stream for additional peak-information on peak-shapes (Tf, w0.5) and peak-names that can be directly used to adjust the model.
Multivariate modeling demand
“The era of new, complex drug products has brought increased demand for multivariate modeling tools: these should yield robust analytical method designs, facilitate an efficient and science-based change management and generally enrich the communication between regulators and industry,” commented Molnár-Institute President, Dr. Imre Molnár.
“Today, more and more efforts are made to improve the concept of Automated Quality by Design (AQbD). This includes, amongst others, missing modern lifecycle aspects, such as long-term usability and performance characteristics of the methods. The understanding of how a method will perform in the long term, routine use is of critical importance today. This is especially true in light of modern production structures that are characterized by high degrees of externalization and frequent method transfers. These imperatives demand highly robust methods that are capable of delivering consistently good results, even if performed in varying geographical and laboratory environments,” said Dr. Molnár.
ICH Q12 guideline compliance
A key advantage of using DryLab®4 for method development is that it can directly call on its huge model-generated database of virtual chromatograms to safeguard methods through later stages, including Stage 2 method performance qualification and Stage 3 routine use. By varying instrument tolerance limits and relevant method parameters, hundreds of chromatograms can be computed in-silico for all possible combination of variables, to forecast results that can be expected during routine use. At the same time, the software can determine the most influential parameters that will shape necessary set point alterations.
Complying with recently introduced ICH Q12 guidelines for assessing the robustness of an analytical method is one of the main reasons, why pharmaceutical companies have turned to automated method development.
“In terms of guidelines, recent developments have introduced the concepts of Analytical Life Cycle Management and its post approval changes (PACMP). These ICH-proposed perspectives grant leeway for continuing improvement to be managed within the design space,” Dr. Molnár explained.
“DryLab®’s Robustness module is well aligned with these principles and the automation module’s click-to-run verification delivers clear answers about the robustness of a method. This can, for instance, verify the six worst resolution cases, or any other approaches that have scientific relevance. Our customers are using successfully this approach in filing QbD-methods in the Common Technical Document. Also, I know several cases, where DryLab® model was preventively built around approved ‘unknown’ methods to avoid any unpleasant surprises later,” he added. [1]
Speeding results by reducing risk
DryLab® is a standalone chromatographic platform that accelerates high performance liquid chromatography (HPLC) or ultra-high performance liquid chromatography (UHPLC) processes by creating in-silico models from cdf or AIA data generated by standard CDS chromatographic data systems.
In particular, it can reduce the risk of out of specification (OoS) results and hugely improve routine method efficiency by integrating Design of Experiments (DoE) and Quality by Design (QbD) disciplines into the process.
DryLab®’s unique gradient capabilities and 3-D resolution maps visually guide researchers in making risk assessments and selecting ideal working points, while the built-in robustness module helps avoid OoS results.
Remote working
The accelerated release of the Empower 3 automation DryLab® expansion module also recognizes the critical time and spatial constraints under which research teams are now working under the pressures of the COVID-19 pandemic.
Empower promises to further facilitate the R&D process to reduce time between drug discovery and approval by optimizing use of instrumentation and determining the most robust method conditions. By reducing costs and ensuring full compliance with FDA/EMA requirements for Method Lifecycle Management, it improves the quality of results and ensures patient safety, while simultaneously reducing Quality Control (QC) costs.
[1] Updating the European Pharmacopoeia impurity profiling method for terazosin and suggesting alternative columns.
Dániel Enesei, Imre Kapui, Szabolcs Fekete, Róbert Kormány
J. Pharm. Biomed. Anal., 187 (2020) 113371
About Molnar-Institute
Founded in 1981, Molnár-Institute develops DryLab®, a software for (U) HPLC modeling for a world-wide market. Its powerful modules allow for the most sophisticated method development as required across pharma industries. Analytical scientists use DryLab® to understand chromatographic interactions, reduce runtimes, increase robustness, and conform to Analytical Quality by Design (AQbD) standards.
Molnár-Institute is registered vendor to the US FDA, CDC and many other regulatory bodies. DryLab® has pioneered AQbD long before regulatory agencies across the world encouraged such submissions. Widely implemented by thought leaders, the software contributes substantially to the paradigm shift towards a science- and risk driven perspective on HPLC Quality Control and -Assurance.
Further information at: www.molnar-institute.com