By Skyepharma Production…
UHPLC : The Analytical Answer to Current Needs
Saint-Quentin-Fallavier, France: – Oral dosage specialist CDMO Skyepharma Production S.A.S. (Skyepharma) has added state-of-the-art ultra-performance liquid chromatography (UPLC) to the range of analytical tools in its analytical development and quality control (AD/QC) laboratory.
Skyepharma’s analytical development and quality control (AD/QC) laboratory has acquired a Waters™ ACQUITY H-Class Plus UPLC ultraperformance liquid chromatograph system that delivers industry-leading performance standards to speed routine analysis and analytical development.
High resolution & sensitivity separation capability
The ACQUITY UPLC H-Class PLUS is a quaternary UPLC system designed for high-resolution, high-sensitivity separations of complex samples with the flexibility and robustness to run all modes of chromatography – ion exchange, size exclusion, hydrophilic interaction, hydrophobic interaction, and reversed phase.
These systems are especially well-suited to, streamlined method development, and for routine analysis laboratories running ultra-high-performance liquid chromatography (UHPLC) analytical methods that are becoming increasingly essential to the pharmaceutical industry for their accessibility and its chromatographic performance.
UPLC advantages
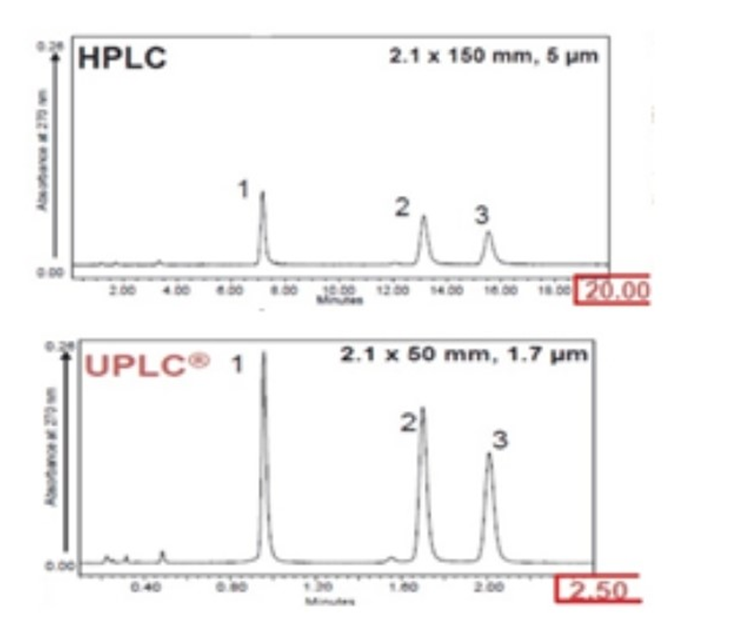
UHPLC systems work on the same principles as HPLC high performance liquid chromatography with a pump delivering a defined flow of mobile phase to a system consisting of injector, column, and detector that separates analytes via chemical interactions between the mobile phase and the stationary phase.
The analytical response translating the concentration of separated analytes is acquired by the UV, or other detector for processing in software, such as Empower 3, used in the Skyepharma laboratory. This software translates the detector response to a chromatogram displaying peak concentrations.
UHPLC improves the method by using thinner tubing, lower injection volume, and very high-pressure resistant pump (up to 15000 PSI) that enable more efficient columns with smaller dimensions (granulometries less than 3 µm, lengths less than 125 mm), while filtering out all particles greater than 0.2 µm in sample solutions and mobile phases. This delivers a number of efficiency benefits, including increased availability of analytical equipment, significantly reduced experiment run times, decreased consumption of reagent and better separation of analytics in mobile phase.
Meeting customer expectations
Skyepharma AD Laboratory Project Manager, Alice Jolibois, says the installation of the H-Class Plus system will enable AD team to meet increasingly challenging customers’ expectations, by offering a wider range of analytical methods and even more precise results.
“Having UHPLC equipment available in our laboratory is a great benefit for our customers. Indeed, analytical method developed with UHPLC are higher performing. The analysis time is lower so is the cost of the analysis with expected quality on results delivered,” Ms. Jolibois explained.
“Moreover, as an analytical project manager, it is a great asset to be able to offer this technology to customers in order to optimize old analytical method as part of the product life cycle management,” she added.
About Skyepharma Production
Skyepharma Production S.A.S. (Skyepharma) is a specialist CDMO with particular expertise and capabilities in oral solid dosage forms, being a key player in drug development and delivery of oral technologies serving the global pharmaceutical, biotech and consumer health industries.
As an integrated CDMO, The company’s vision is to help solve healthcare industry complexity with a mission to provide a dedicated and results-oriented team to deliver advanced oral dosage services to the healthcare industry through state-of-the-art facilities, scientific expertise and open, transparent relationships. All of this is summed up in its strapline: ‘Expert and Agile CDMO partner for tailor-made solutions’.
The Skyepharma CDMO offer in solid oral dosage formats encompasses the whole value chain, including Formulation Development & Process Design, Analytical Development, Scale-up, Manufacturing, Packaging, Serialisation and Aggregation.
Skyepharma also provides a range of support services that help client companies from early stage development (up to phase III), through scale-up and full commercial manufacturing and packaging to market introduction, including controlled substance handling, QbD methodology (FMEA, FTA, DOE), Troubleshooting, regulatory services, validation, registration and warehousing services.
Learn more at: www.skyepharma.fr
Resources
Click on UHPLC : The analytical answer to current needs for more information.