By Skyepharma Production…
Skyepharma shares insights on mastering process scale-up challenges
Saint-Quentin-Fallavier, France: – The latest Insights technical article from oral dosage specialist CDMO Skyepharma Productions S.A.S. (Skyepharma) focuses on the scale-up rules that it applies in the industrialisation of wet granulation and powder compression processes to ensure flawless scale-up from lab development to full production and get it ‘right first time’.
Technical transfer from small scale development formulation to industrial scale manufacturing method poses major issues for oral solid dose (OSD) producers with manufacturing of pharmaceutical tablets a complex process involving several steps, notably powder granulation and compression, in which various problems can occur.
QbD principles
The ultimate goal of Skyepharma’s development teams is to identify robust manufacturing processes that guarantee product quality.
One of the first steps of the pharmaceutical development is based on Quality-by-Design (QbD) principles to explore the process “frontiers” and to determine which parameters are critical to obtain a high quality product. This involves a series of experiments executed at labscale in order to minimise API consumption and optimise operations duration.
However, since batch size itself influences process success, the main challenge is to ensure work done at lab scale is truly representative of production scale.
Powder granulation
The wet granulation is commonly used to improve the flowability, compressibility and the stability of pharmaceutical powders in numerous cases, leading to process facilitation. The high shear granulator simply consists of a bowl and a rotor at the bottom that mix powder and helps densification all along wetting. The granulators are also often equipped with additional chopping knives used to break up large agglomerates.
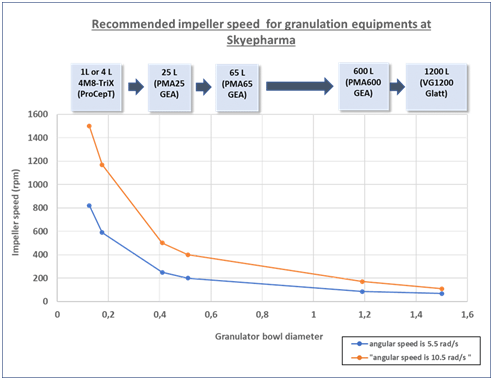
During process scale-up, the critical granulator parameters must be considered to ensure consistent and reproducible results at any scale, such as filling level, impeller speed (speed of rotor and total revolutions set on the equipment), wetting flow rate, homogenisation duration, chopper speed, and overall liquid amount.
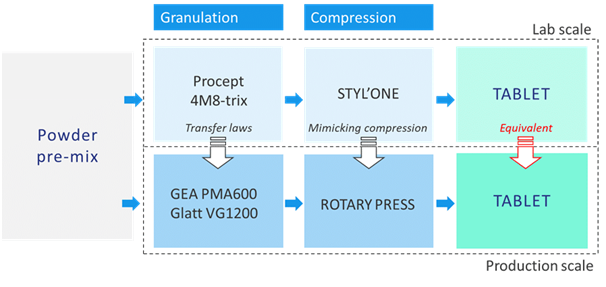
Skyepharma is able to perform granulation coupled with fluid-bed drying from lab scale (up to one liter) to industrial scale (600 to 1200 liters). At granulation scale-up, some parameters have to be maintained as constants to ensure final batch-to-batch consistency of compressibility. These parameters include granulator bowl filling level, wetting flow rate (amount of liquid sprayed/min/kg of dry inner phase) as well as the angular velocity (tip speed of rotor), which is a function of impeller speed and bowl diameter. Skyepharma thus needs to specify impeller speeds on each piece of granulator equipment to ensure successful scale-up.
Transfer protocols
Developing robust granulation processes at lab scale relies heavily on the knowledge and expertise of Skyepharma’s granulation team and well-defined transfer protocols laws in dealing with product variations and anticipating scale up parameters. Small scale process allows the team to provide expertise and to suggest improvements in order to perform the full-scale process right first time at lower cost and consumption of raw materials.
The main critical point at blend preparation is to ensure that the active ingredient is uniformly distributed into the final blend to be tableted. This is achieved by maintaining same number of revolutions (speed x mixing duration) at mixing during lubrication and final blend preparation steps.
The final blend must also present suitable porosity in order to get tablets with targeted hardness, dissolution, disintegration and other quality properties. Skyepharma’s helium pycnometer instrument helps to compare different blends and ease pharmaceutical product development.
Powder compression
The series manufacturing of tablet is performed on rotary presses, for instance on Hata, Fette or Kilian machines at Skyepharma. These platforms have impressive production rates up to tens of thousands tablets per hour, consuming high quantities of granulated powder.
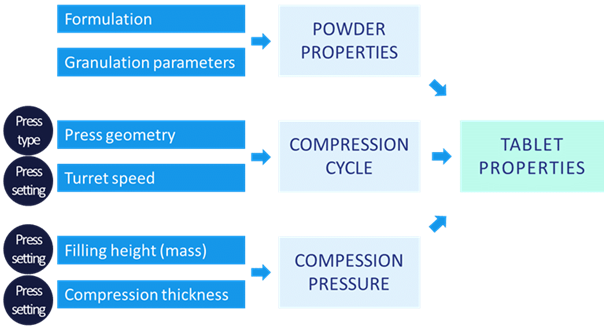
The properties of the final tablets from a rotary production press are mainly driven by intrinsic powder properties (determined by quality of excipients and granulation process), compression pressure and the compression cycle and speed, particularly the position of the punches according to the time during the compression step. These latter two determinants are governed by rotary press type and settings.
If any part of this is incorrectly done resultant problems and tablet defects can include incorrect mass, poor release properties, lamination, chipping or sticking.
STYL’One modelling
To accurately develop a tablet that avoids these problems, without expensive time on a full rotary press, the Skyepharma developer teams perform their compression experiments use the STYL’One Evolution development tableting simulator. This machine from Lyon-based Medelpharm is a single station tablet press that is instrumented and numerically controlled using incremental sensors. This device allows development teams to mimic the geometry of the rotary press that will be used for the large-scale production, and replicate all combinations of press settings. The whole tableting process can accurately modelled as on the STYL’One to form a perfect reproduction of the compression on the large-scale machine, while using very low quantities of active ingredient and other materials.
Thus, the optimal parameters settings are determined in lab scale on the STYL’One, and can be precisely transposed to the large-scale to obtain the same compression step, resulting in tablets with the aimed properties and attributes. It is particularly useful to plot a hardness plateau or to define rejection limits to be applied on the industrial press.
Conclusion
With their fine expertise in granulation and compression operations, Skyepharma’s teams are able to upscale processes from laboratory to industrial scale with very high consistency, predictability and overall success rates.
By using transfer laws in initial granulation and precisely modeling compression cycles in powder compression, it is possible to reach the “right first time” at process scale-up, thus saving time and money for Skyepharma customers.
About Skyepharma Production
Skyepharma is an independent French pharmaceutical CDMO, 100% owned by its management team and Bpifrance. Skyepharma is an expert CDMO specialised in the formulation, development and manufacturing of complex oral solid forms (OSD), with a specific expertise and proprietary technologies on modified release products.
Skyepharma is based in Saint-Quentin-Fallavier, France. The current factory, dedicated to its OSD activity, occupies 22,000m2, on a 60,000m2 piece of land. Skyepharma has decided to allocate a portion of the available land (more than 20,000m2) to establish its SkyeHub Bioproduction, an innovative model designed to offer clinical and commercial production capacities to biotech companies. This SkyeHub model includes the construction of dedicated buildings, with specifically designed surfaces and premises, together with transverse support services such as quality, maintenance, batch release, etc.
Learn more at: www.skyepharma.fr
Resources
Click on Process scale-up: how to ensure right first time? to access published article.