By Molnár-Institute for…
MOLNÁR-INSTITUTE collaborates on updating old European Pharmacopeia method
Berlin: – The research team of University of Pannonia and Egis Plc in Hungary has recently introduced a workflow featuring powerful in silico modeling capabilities to quantify and compare stationary phase selectivities. This methodology was also verified in a subsequent proof-of-concept study resulting in the modernization of a European Pharmacopeia method of cetirizine.
Assessing Column Equivalency
The recently published study ‘Updating the European Pharmacopoeia impurity profiling method for cetirizine and suggesting alternative column, using design space comparison’ showcases a real-life application example on a complete method redesign of an outdated European Pharmacopeia (EP) method.
The original EP-compendial method for cetirizine impurity analysis defined a bare silica hydrophilic interaction liquid chromatography (HILIC) column, making it less attractive for routine applications. Instead of anticipated future troubleshooting efforts, a generic design space methodology was applied to better understand the separation possibilities offered by a number of commercially available reversed-phase (RP) columns. By acquiring 2-dimensional design space models, single, as well as shared separation areas—intercolumn Method Operable Design Regions (MODRs)—of several C18- and PFP-stationary phases were systematically explored. The obtained modeling knowledge allowed science-based decision-making process to specify a primary stationary phase and compare it with other columns offering similar or equivalent performance.
The focus is on Comparing Design Spaces
The topic on identifying interchangeable columns has always seen great academic and industrial interest, dating back as far as the conception of HPLC. However, most of the developed traditional testing methodologies often fall short of reliably accounting for column equivalencies. Focusing on overcoming these limitations the work draws on the results of earlier papers of this group. This includes published examples of a leading-edge computer modelling to optimizing column selectivity [1], simulating robustness testing [2] and assessing column interchangeability [3].
In this current instance, the team set out to actualize the method described for cetirizine related substances in the European Pharmacopoeia monograph. This included the development of a generic workflow to evaluate resolution changes in a full design space context and use this knowledge for new method specification workable on multiple columns. The applied methodology was hoped to serve as a generic working scheme for other HPLC innovators and separation experts to find new, more efficient ways of analytical procedure developments.
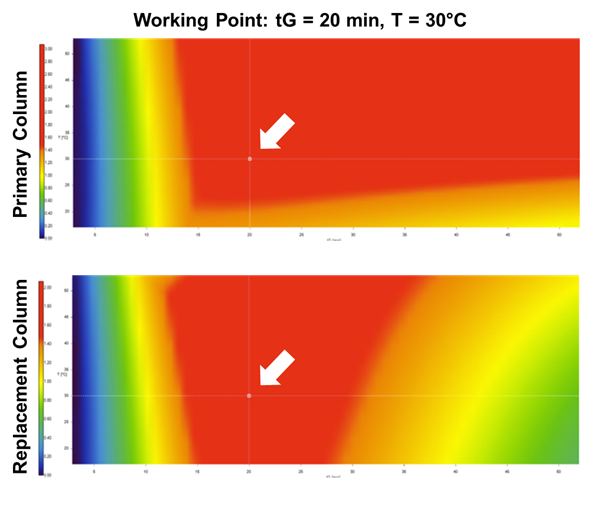
To attain this objective, they performed a small number of experiments with just 4 input runs to generate simple 2-D gradient time-temperature (tG-T) design space models. The team used DryLab®4 software as the modeling tool, taking advantage of the recently added Design Space Comparison Module (DSC) that allowed researchers to overlay the 2-D resolution maps of the modelled columns to identify shared MODR areas, where two or more stationary phases meet the same given resolution criterion. This granted the essential knowledge to specify all the suitable run conditions that would provide with comparable or virtually equivalent separation performance on the selected stationary phases, as shown in the figure below.
Science-based Decision Making
While the selected stationary phases showed obvious differences in their design space test results, two of the six columns shared MODR-spaces, indicating that under the outlined conditions they were conveniently interchangeable. At last, a final robust working point within the MODR was selected and a model-based robustness study along with full-method validation performed.
“During method development, it is recommended with such a method to look for a suitable interchangeable stationary phase,” the team comment in their conclusions.
“Finally, a single robust method was developed and validated in accordance with the requirements of the pharmaceutical authorities and proposed to update the old pharmacopoeia method,” they add.
Future Perspectives
As the study concluded, the Design Space Comparison feature of the modeling tool is highly applicable for finding alternative stationary phases for any given separation examples, rather than commonly used column test procedures. If shared areas of column design spaces exist, they can reliably be exchanged without the compromise of an insufficient resolution or robustness. Quite analogously, this approach can further be extended to compare competitive phases or to verify batch-to-batch reproducibly of columns—as demanded by regulatory bodies in order to enhance the analytical procedure’s robustness.
References
- Róbert Kormány, Katalin Tamás, Davy Guillarme, & Fekete, S. (2017). A workflow for column interchangeability in liquid chromatography using modelling software and quality-by-design principles. Journal of Pharmaceutical and Biomedical Analysis, 146, 220–225. https://doi.org/10.1016/j.jpba.2017.08.032
- Kormány, R., Fekete, J., Guillarme, D., & Fekete, S. (2014). Reliability of simulated robustness testing in fast liquid chromatography, using state-of-the-art column technology, instrumentation and modelling software. Journal of Pharmaceutical and Biomedical Analysis, 89, 67–75. https://doi.org/10.1016/j.jpba.2013.10.029
- Róbert Kormány, Imre Molnár, & Rieger, H.-J. (2013). Exploring better column selectivity choices in ultra-high performance liquid chromatography using Quality by Design principles. Journal of Pharmaceutical and Biomedical Analysis, 80, 79–88. https://doi.org/10.1016/j.jpba.2013.02.028
About MOLNÁR-INSTITUTE
Founded in 1981, Molnár-Institute develops DryLab®4, a software for UHPLC modelling for a world-wide market. Its powerful modules gradient editor, peak tracking, automation, robustness and Design Space Comparison allow for the most sophisticated method development as required across modern pharma industries. Analytical scientists use DryLab®4 to understand chromatographic interactions, to reduce analysis time, to increase robustness, and to conform to Analytical Quality by Design (AQbD) principles, according to the recently published ICH Q14 regulatory framework.
The Molnár-Institute is a registered partner of the US-FDA, CDC and many other regulatory bodies. DryLab®4 pioneered AQbD long before regulatory agencies across the world encouraged such submissions. Widely implemented by thought leaders, the software contributes substantially to the paradigm shift towards a science and risk driven perspective on HPLC Quality Control and Assurance.
Further information at: http://www.molnar-institute.com/
Resources
Click on Updating the European Pharmacopoeia impurity profiling method for cetirizine and suggesting alternative column, using design space comparison to access the full study.