By Bachem AG
Bachem’s journey to Industry 4.0 manufacturing
Bubendorf, Switzerland: – Bachem, the leading CDMO for peptides and oligonucleotides manufacturing, has begun its transition to Industry 4.0 manufacturing with its concepts of the ‘smart factory’ featuring automation, digitalization and data-driven integration of machines and processes.
The first step on the journey is to automate and digitalize one of Bachem’s core business processes, solid phase peptide synthesis (SPPS), redesigning operations so that they can be carried out with minimal human intervention, thus improving the reliability of the process, reproducibility of results and safety, while significantly increasing the cost-effectiveness of operations.
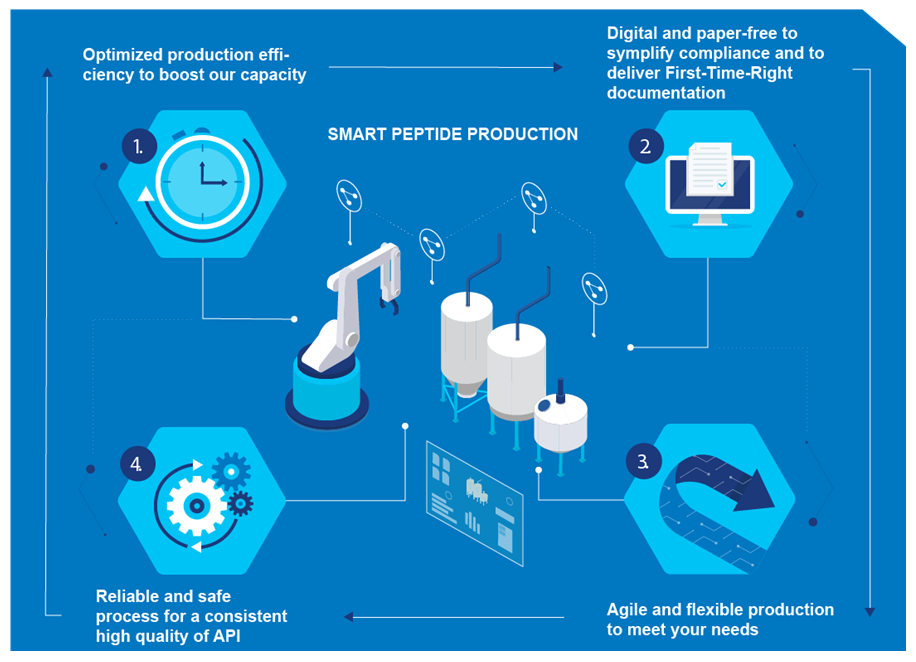
This has already allowed Bachem to optimize equipment utilization and reduce operating times and personnel time to a fraction of previous levels, releasing higher capacity, efficiency and agility of production along with more flexibility to take on new projects. Bachem has also invested in digitalization of the production process so that documentation can be conducted in a paper-free way. Furthermore, it can now attain improved first-time-right (FTR) rates, along with data analytics by implementing a plant information (PI) system, and higher data safety and integrity.
Industry 4.0 concept
Industry 4.0, or the fourth industrial revolution, refers to the automation and digitalization of traditional industrial processes through smart technology and integration. Within this concept, a “smart factory” is characterized by machines which are interconnected, and interoperable, and can process data autonomously. Such “smart factories” require only limited human decision-making or intervention and are therefore sometimes referred to as “intelligent automation.”
Innovation and operational excellence are two important pillars of Bachem’s success. Thus, to keep technological leadership and high-quality services, it was essential to start its digital transformation and to enter the Industry 4.0 area. This will benefit customers, through increased capacity with a more agile and flexible production, simplified and improved GMP documentation, and a safer, more reliable, and scalable manufacturing process delivering consistently high quality API active ingredients.
Process innovations
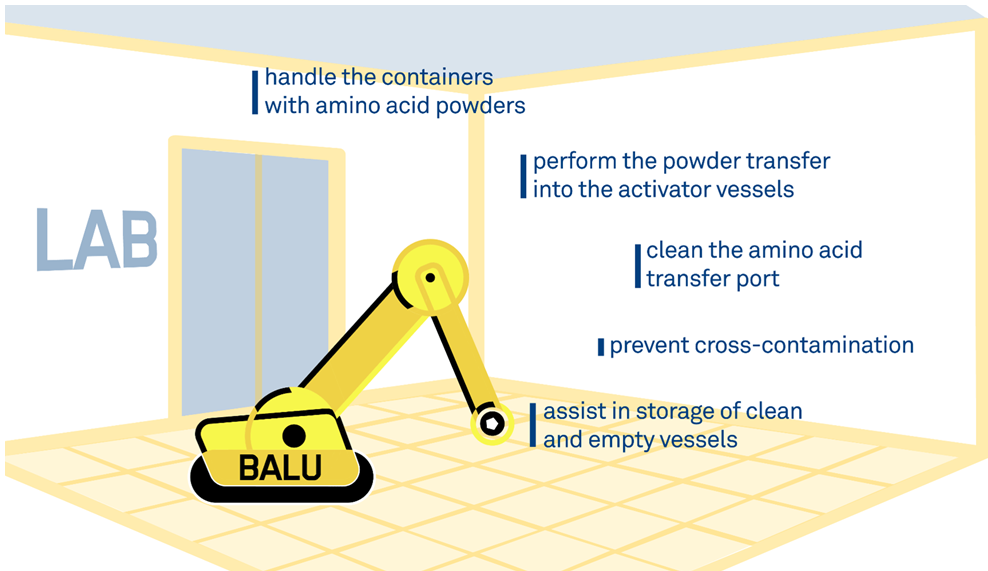
The transition to ‘smart factory’ has involved some major innovations at Bachem, including the introduction of its first robot-operator, BALU. This machine is designed and programmed to support commercial scale of SPPS. BALU handles containers for amino acid powders and perform powder transfer into the activator vessels for 150L SPPS reactors without any need for operator involvement of an operator. BALU can perform other critical tasks, such as cleaning the amino acid transfer port to prevent cross-contamination. A barcode scanner that reads the labels placed on the amino acid containers ensures correct handling.
A further innovative feature of the new fully automated SPPS process is Process Analytical Technology (PAT), which performs inline analytics after key steps. PAT removes the need for manual In-Process Controls (IPC) and provides a better control of Critical Process Parameters (CPP). Additionally, this automated process enables data recording and analytics as well as paper-free cGMP documentation.
Bachem’s implementation of PAT in its process control decreases human contributions and cost of goods. Indeed, manual tasks are no longer required, freeing human resources for other tasks and projects. Furthermore, PAT leads to a higher reproducibility with minimized chemical side reactions. Ultimately, Bachem is able to utilize assets more efficiently and streamline production scheduling for a higher capacity and flexibility.
Digitalization Strategy
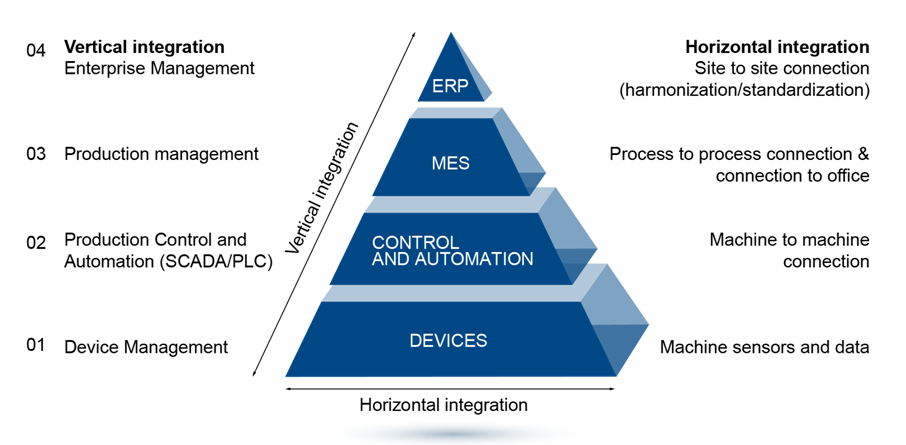
Bachem uses a pyramid concept to guide its digitalization of the SPPS process. Machines and devices are connected horizontally while processes are integrated vertically, starting with devices at the base, integrated via SCADA and PLC control and automation systems to a Manufacturing Execution System (MES) that defines the sequence of operations that have to be performed guided by a Master Batch Record (MBR). The MES also records all events, process values, alarms, as they happen during the process, and finally generate the electronic Batch Report, eBR. The MES allows all equipment to be managed without need for physical logbooks. This paperless documentation is fully GMP compliant.
Full digitalization is achieved by connection between MES to the ERP (Enterprise Resource Planning) system from SAP at the apex. This effectively connects production to market with automated executions of process orders, automatic take-out and stock creation, material flow and inventory controls that are also fully automated and paperless.
The final elements of Bachem’s digitalization strategy include logging in real time and long-term archiving of data on a Historian platform. This PI system allows for real-time and remote monitoring of the factory floor and easy access to data for trending and batch-to-batch comparisons. This provides the basis for future predictive and prescriptive data analytics.
Towards a smart future
As a CDMO, Bachem sees implementation of new levels of automation and digitalization as necessary to meet the predicted rise in demand for capacity and compliance. These demand boosts to process efficiency in time, cost, supply and distribution. Entering industry 4.0 and developing our “smart factory” represents a big step forward for Bachem customers in ensuring swifter interaction and more flexibility in manufacturing and in documentation sharing.
As digitalization and automation initiatives multiply across the pharmaceutical industry , Bachem’s pioneering experiences in SPPS will enable it to transform a growing number of processes in this way. With these innovations, Bachem aims to retain its technological leadership and set world-class industry standards for the benefit of its customers.
About Bachem
Bachem is a leading, innovation-driven company specializing in the development and manufacture of peptides and oligonucleotides.
With over 50 years of experience and expertise Bachem provides products for research, clinical development and commercial application to pharmaceutical and biotechnology companies worldwide and offers a comprehensive range of services.
Bachem operates internationally with headquarters in Switzerland and locations in Europe, the US and Asia. The company is listed on the SIX Swiss Exchange.
For further information, see www.bachem.com.
Resources
Click on Implementation of Industry 4.0 concepts for ‘tides manufacturing to watch full presentation via Bachem360 (Visit CMC development section inside the live area).
Click on Bachems News to see latest News & Events.