By MEGGLE Excipients
MEGGLE discovers advantages for co-processed lactose and starch in ODT formulations
Wasserburg, Germany: – Pharmaceutical lactose specialist MEGGLE Excipients & Technology (MEGGLE) has conducted a research study to gauge the suitability of its StarLac® co-processed lactose/starch formulation as a robust excipient for orodispersible tablet (ODT) applications.
The study has found that StarLac® possesses significant stability and economic advantages, together with more pleasant ‘mouth feel’ for the patient.
ODT Trends
The study StarLac® for Orally Disintegrating Tablets: What can lactose and starch do for an ODT formulation? was undertaken by a research team from MEGGLE Excipients & Technology’s São Paulo Laboratories, headed by Dr. Carolina Paz de Almeida, supported by Dr. Franz Penz, Fábio Luis Ikuno, Guilherme Brandão, and Sabine Hauptstein.
The study is a response to a recent upsurge in preference for ODT formulations over conventional oral tablets (OCT), driven by patient benefits for infant and geriatric groups as well as in applications like schizophrenia and dysphagia medications, where patients have difficulty in swallowing.
As a 2011 report by the National Institute of Aging remarked, “Taking this and the increasing world population of elderly people (16 %) into account, the increasing interest in ODT is explained easily.”
However, as Annex 5 of the World Health Organization (WHO) Expert Committee on Specifications for Pharmaceutical Preparations report on development of pediatric medicines has pointed out, disintegration characteristics are not the only important factors in formulation, which must also consider affordability, ease of production, transportation and storage.
Manufacturing challenges
While freeze-drying, molding and sublimation, spray drying and vacuum drying have all been used for ODT manufacturing, the most common process remains direct compression (DC).
The physical properties of orodispersible tablets are also affected by proportion of superdisintegrants, effervescent agents, highly compressible diluents and binders, along with combinations of ion exchange resins and cyclodextrins.
The overall manufacturing challenge is to achieve the optimum balance of rapid disintegration, pleasant taste, mechanical resistance, stability and mouth feel for the patient.
StarLac® characteristics
Since 2002, MEGGLE’s StarLac® co-processed excipient, composed of 85 % lactose monohydrate and 15 % maize starch, has proven itself as a robust excipient with favorable fast drug release properties.
The aim of the latest study was to explore to what extent StarLac® can also form an innovative solution for the ODT market.
The researchers made three-way comparisons between placebo tablets of StarLac® against a StarLac® based formulation containing nonsteroidal anti-inflammatory drug (NSAID) Ketorolac tromethamine as model API, along with Ketorolac formulation using polyols sorbitol and mannitol commonly used as excipients in ODT formulations in combination with disintegrant.
For all investigated formulations, three different packaging options were compared: PVC/aluminum blister, PVDC/Aluminum blister and Amber Glas flask with plastic cap.
The tablet/packaging combinations were then subjected to an accelerated stability study with placebo tablets stored at 40°C / 75 % relative humidity (RH) for three months compared with six months for formulations with API, Physical analyses for weight, hardness, disintegration and friability were carried out at start, and after one, three and six months
Study results
Findings included:
- Tablet weight: the formulation using only StarLac® as filler/binder showed limited moisture absorption, independent of packaging material, while ODT formulation based on polyols (FAP) showed an increase in tablet weight due to moisture uptake, particularly when packaged in PVDC blister.
- Tablet hardness: Placebo formulation with StarLac® demonstrated less hardness loss at three months compared to the Mannitol/Sorbitol formulation, which could only maintain certain hardness when packed in glass flask. For standard ODT formulation stored in blister packaging it was not possible to assess hardness at all planned time points because the tablets were not stable enough to be transferred to hardness tester.
- Tablet disintegration: Again, for standard ODT formulation based on polyols it was not possible to investigate disintegration characteristics since tablet hardness had so decreased at three months (PVDC) or six months (PVC) as to make them impossible to transfer from blister intact. StarLac® based tablets in all packaging formats fulfilled European Pharmacopoeia (Ph.) requirements for ODT disintegration within three minutes. Standard polyol-based formulations fulfilled requirements initially but then lost physical stability during storage.
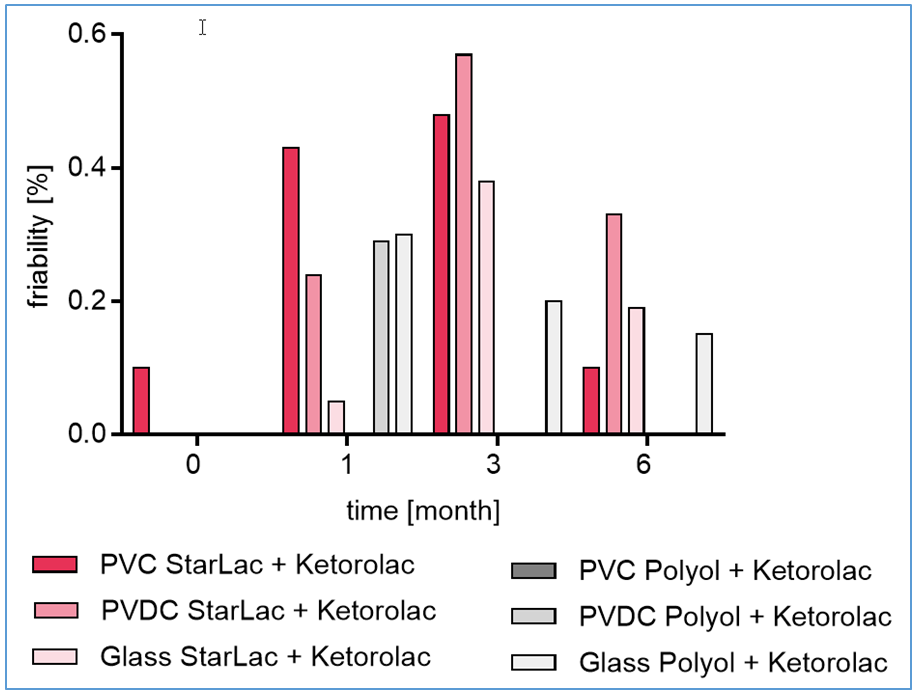
- Friability: The standard ODT formulation showed increased friability, again highly dependent on packaging, with tablets stored in glass showing slight decrease in friability whole those stored in blister packs were not stable enough to be transferred to friability tester at all planned time points. For all investigated StarLac® based formulations and the standard ODT formulation stored in glass bottles, friability below 1 % was observed over the complete time-frame.
Study conclusions
The tests showed StarLac® as exceptional in providing a robust excipient for ODT applications, presenting stable results for weight, hardness, disintegration and friability when submitted to stability study at 40 °C / 75 % RH, independent of packaging formats.
When formulated with API and other excipients like sweetener and aroma, StarLac® ODT has proved to be less prone to instabilities compared to polyol-based formulations, allowing manufacturers greater headroom to package final formulation in PVC-Aluminum blisters.
StarLac® can provide important benefits to pharmaceutical companies provided chosen API does not show any degradation due to moisture. With StarLac®, it is possible to achieve a faster development process, a robust formulation for a trouble free production process with fewer components, resulting in a more economic formulation overall and allowing for the use of the most economic packaging format.
There are also patient benefits in resistant tablets that do not break when pushed out of blisters, that disintegrate rapidly in in the mouth, and that provide a pleasant mouth-feel without “sandy” sensation, raising adherence rates.
The research study indicates that lactose and maize starch, two of the best established and safest excipients on the pharma market, have promising continuing roles in ODT formulations when co-processed as StarLac®.
About MEGGLE
Bavarian-based MEGGLE is one of the world’s experts in lactose-based excipients and powder technology.
From its roots as a dairy operation in the late 1880’s, MEGGLE has become one of the world’s leading manufacturers of pharmaceutical lactose, supplying the pharma market segment with a broad-based and unique lactose product portfolio.
MEGGLE Excipients & Technology has harnessed outstanding product quality and intelligent innovation to become a global leader in the manufacture of lactose-based excipients, focusing on products for direct tableting and dry powder inhalation.
A multidisciplinary team of committed and highly qualified people allows MEGGLE clients to benefit from pioneering experience and innovative drive in industrial milk and whey processing. The company constantly strives to develop high-tech, functional products for solid dosage forms and DPI applications, where they can deliver maximum performance.
The company has introduced several pioneering products, notably Tablettose®, the world’s first agglomerated lactose for direct tableting, Cellactose® 80, a pioneering co-processed excipient based on cellulose-lactose for outstanding compression and flow properties and RetaLac®, the first direct compression co-processed excipient using a hypromellose-lactose base for sustained release formulations.
Further information at: https://www.meggle-pharma.com