By Skyepharma Production…
Skyepharma’s Quality by Design (QbD) approach
CDMO solutions provider, Skyepharma Production S.A.S. (Skyepharma) can help its clients implement a thorough Quality by Design (QbD) approach throughout their entire drug development and manufacturing programs.
By embracing QbD disciplines, methodologies and accessible tools in its own operations, Skyepharma can assist clients in building in quality right across the development value chain, from early-stage formulation design to commercial production.
QbD disciplines
Skyepharma now embraces a systematic global quality approach emphasizing QbD disciplines and guidelines from the earliest stages of development.
Quality by Design is defined in the International Council for Harmonization (ICH) Q8 guideline by “A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.”
Skyepharma encourages its partners to adopt this approach from the beginning of the project to promote shared understanding from the outset of the key formulation and process parameters that will impact critical quality attributes of the developed product. This greatly speeds development through to obtaining a first qualified prototype, with even greater acceleration of the subsequent scale up and industrialization steps.
Furthermore, the approach facilitates easier approval of submission files by the regulatory authorities.
To support customers in their drug product development projects, Skyepharma has reinforced its teams to expanded expertise in the key areas of design and test, DoE (Design Of Experiments), and quality assurance in manufacturing.
Design and test
As a first step in the process, the customer and Skyepharma will jointly establish a Quality Target Product profile (QTPP) that defines the efficacy and safety requirements of the medicinal product necessary for market authorization (MA). Establishing this product’s “identity card” is an essential prerequisite to the implementation of QbD.
During development phase, Skyepharma project teams will define the Critical Quality Attributes (CQA) of the drug product, along with its potential Critical Material Attributes (CMA) and Critical Process Parameters (CPP) that may impact CQA, such as API density, water amount during granulation, blend particle size distribution or even the permeability of blister packaging.
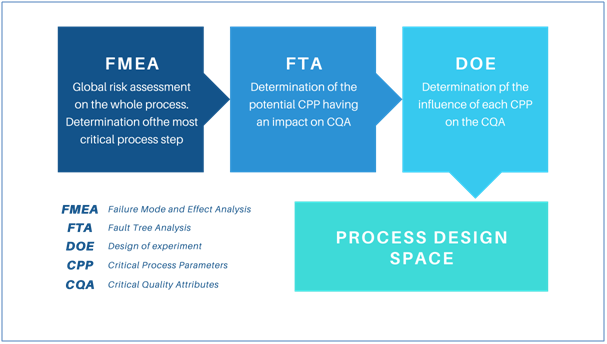
Criticality of identified CMAs and CPPs on product quality is identified by drawing up a risk analysis (FMEA : Failure Mode Effect Analysis is a common tool used at this step) for each process step to quantify the impact of each of them on drug product CQAs, using an internal scoring and eventually determine cumulative criticality for each manufacturing or packaging process step.
DoE in the Skyepharma QbD process
The Skyepharma QbD approach emphasizes identification of all sources of variability, whether they are dependent on the material used, on the formulation or the process itself. This contributes to more efficient scientific R&D approach that seeks to obtain maximum results with fewer tests, using experiment matrixes designed to cover the entire spectrum of variabilities and manufacturing steps. This Design of Experiments (DOE) methodology allows researchers to highlight impacts of the different parameters studied on final product quality.
To deliver robust results, this approach may require the manufacture of a certain number of batches. This can be API-consuming, even when performed at pilot scale. Skyepharma has the capability to manufacture at labscale (0.5-1.0 kg), replicating every stage of the eventual manufacturing process from granulation to final coating.
Where Skyepharma is undertaking original research and development on behalf of a client, QbD may be applied to establish the optimal qualitative and quantitative formulation, using experimental designs to vary nature and proportions of active ingredient and excipients.
Quality assured manufacturing using design space
The DOE results in the definition of a formulation/process Design Space. According to ICH Q8, this space comprises “input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality” of the final drug product.
With the design space defined, it is possible to conduct stability studies of the bulk or packaged product.
At this stage, the robustness of the process with respect to the parameters considered critical is already ‘designed in’. The next challenge is to maintain this robustness across different environments during the transition from laboratory to industrial scales. It is therefore necessary to analyze each step of the process in order to determine the limits imposed by tooling, comparing these with the limits of the manufacturing process Design Space, setting appropriate NOR (Normal Operating Range) and PAR (Proven Acceptable Range) limits.
Skyepharma QbD benefits
Skyepharma’s Quality by Design methodologies deliver tangible customer benefits:
- Smoother regulatory process: Working within ICH Q8 guidelines and Design Space concepts result in reduced delays in processing post-approval submission files and the possibility of process improvement while remaining within original Design Space, eliminating need for additional controls to be approved by the relevant authorities. In short, QbD approach delivers enhanced operational flexibility. Furthermore, the Skyepharma methodology also complies with the ICH Q12 directive governing Pharmaceutical Product Lifecycle Management.
- Reduced development time and costs: The Quality by Design approach to a new product can at first glance increase the length of the early-stage development phase. However, the management of the means and tools to be put in place allow an optimization of the working time, thus saving time and money later on.
- Improved lead times for commercial batches: QbD compliant production applies controls throughout the life cycle of the drug, acting to reduce the number of “failures” and rejected batches. In addition, the waiting time for test results is reduced, which shortens the manufacturing time and thus promotes faster real-time batch release. Thanks to the scientific knowledge acquired as well as the traceability of all data generated during development, it becomes easier to trace the cause of any complaint or routine production problem.
Skyepharma’s expertise in oral drug development and labscale platforms can help its clients increase knowledge of their products and boost their development phase using less materials and resources. Now, its customized QbD approach allows customers to benefit further from innovative solutions to optimize testing efforts, accelerate execution and reduce development costs.
Resources
Click on Quality by Design at Skyepharma to learn more.