By Pharmatrans SANAQ…
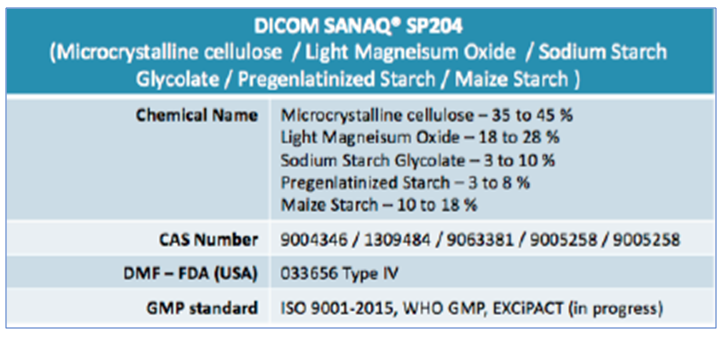
Pharmatrans DICOM SANAQ SP204 proprietary excipient for Direct Compression containing alkaline agent
Pharmatrans Sanaq has extended its DICOM SANAQ® range of high functionality co-processed excipients with the introduction of DC® SP 204 as a co-processed excipient optimized for direct compression and containing an alkaline agent. This makes it especially suitable for acid sensitive APIs.
DC® SP 204 incorporates a large surface area of Light Magnesium Oxide to provide enhanced protection and stability to acid labile active pharmaceutical ingredients from a 360o alkaline environment.
DICOM-DC® SP204 Features
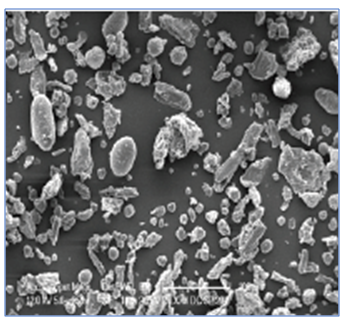
DICOM-DC® SP204 is a proprietary co-processed excipient containing Microcrystalline Cellulose, Light Magnesium Oxide, Sodium Starch glycolate, pre-gelatinized starch and Maize starch.
The presence of the alkaline microenvironment is essential in case of The large Magnesium Oxide surface area in DICOM-DC® SP204 creates a 360° alkaline microenvironment at pH 9.5-11.0. This is essential in formulations involving acid labile drugs (e.g. Pantoprazole) to provide enhanced protection to the active ingredient.
DICOM-DC® SP204 also exhibits high bulk density making it suitable for formulation of high weight tablets.
DC® SP204 Benefits
As part of the Pharmatrans mission to produce ‘Pharmaceutical Excipients focused on your formulation needs’, DICOM-DC® SP204 is engineered to provide directly identifiable and quantifiable user benefits.
In particular, the incorporation of Light Magnesium Oxide into the co-processed excipient overcomes a number of previous problems.
-
- Light Magnesium Oxide is a very fluffy material that is difficult to incorporate directly into a tablet formulation
-
- Segregation tendency leads to weight variations, unstable formulations, and a non-uniform alkaline microenvironment
-
- The inconsistent granular mix can lead to improper die filling and tooling damage without special coatings.
In contrast, use of DC® SP204 offers:
- Uniform alkaline protection from all sides
- Direct compression at high speed with uniform weight and quality attributes
- Enhanced stability for acid labile APIs
- Higher product quality in being able to form robust tablets with reduced friability
- Reduced tooling wear and damage through indirect contact and protection from other excipients in the co-processed mix
- No special coating requited for tooling
- Hugh bulk density allows formulation for high tablet weight using smaller die or high fill weight in smaller capsules


Resources
Click on DICOM SANAQ portfolio for a summary of available blends.