By Gasporox AB
Gasporox introduces GPX HMI 21CFR Part 11 compliant software with extended functionality
Lund, Sweden: – Non-destructive testing technology specialist Gasporox AB has rolled out its new GPX HMI software for extended functionality operations of its testing instruments and sensor modules in GMP-regulated environments.
The new software package meets the needs of pharma industry customers for operating solutions that comply to with Good Manufacturing Practice (GMP) and Good Automated Manufacturing Practice (GAMP) guidelines. In particular, this requires software that meets CFR21 Part 11 demands for full security and traceability of data and electronic records and cGMP requirements for full Audit Trail functionality.
Advanced HMI features
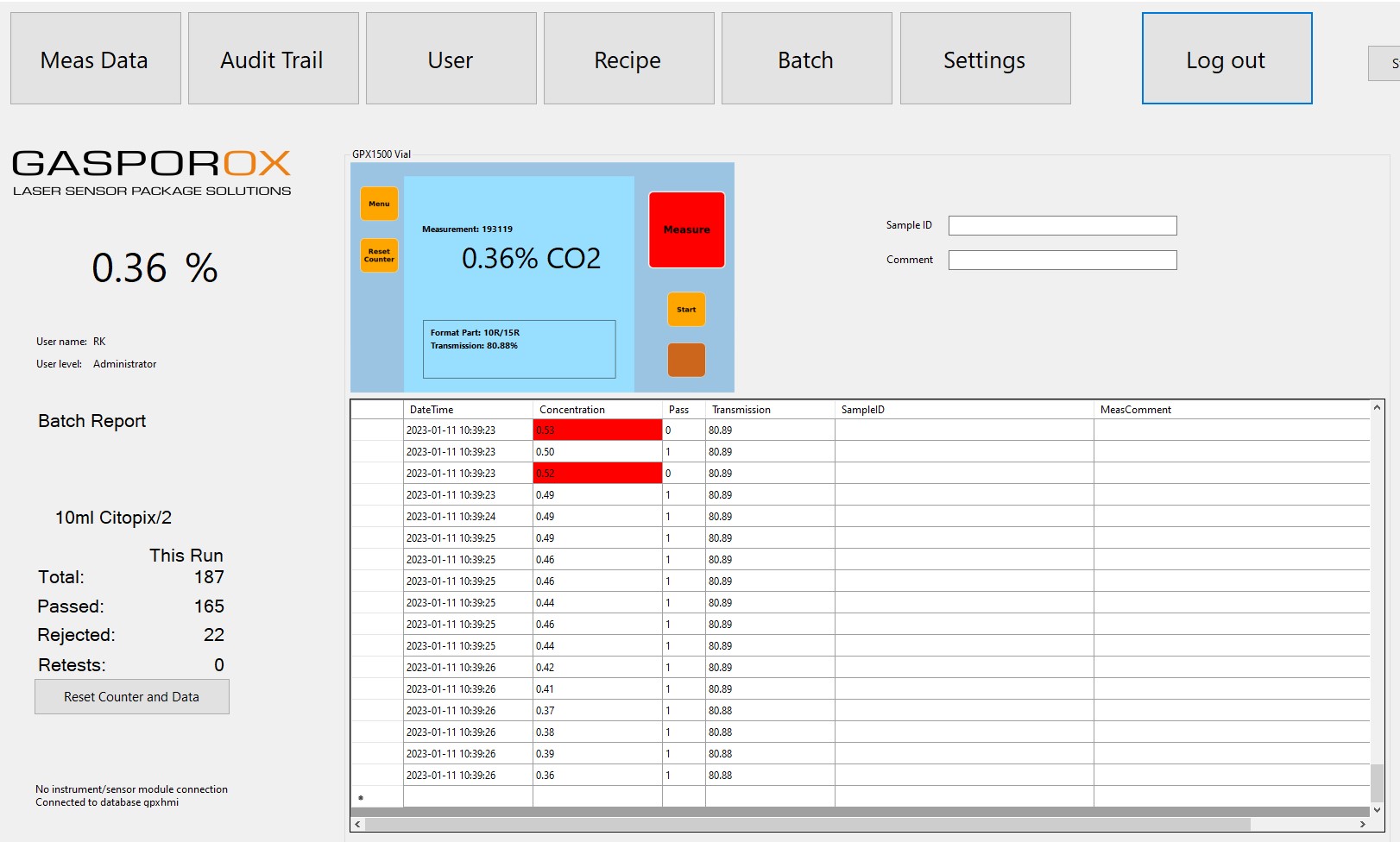
The GPX HMI software package supports all Gasporox Instruments, such as the GPX 1500 series of vial and film testers, and its VialArch™ and other sensor modules that can be installed on production lines for CCIT container closure integrity testing.
The new software manages all data in an SQL database as electronic records, with full recording of traceable datasets to meet the CFR21 Part 11 guidelines on compliant electronic records. The HMI software also features configurable user management with three levels of access privileges, along with recipe management with automatic versioning and traceability within batch reports that also include batch summary, audit trail and measurement data as protected pdf files.
HMI forms a valuable and highly accessible supplement to the existing Excel®-based Gasporox Report Generator software, but with significantly greater functionality for batch handling and reporting, recipe management and Audit Trail functionality.
Complete solution
Gasporox Senior Application and Sales Manager Roland Koch commented: “Our development of GPX HMI meets latest pharmaceutical regulations that require GMP compliant software tools for Electronic Records and Electronic Signatures.”
“We were also seeing increasing industry demand for a complete solution from Gasporox: one that would include one stop supply, easy implementation of the software with no extra interface or other software needed and just a single supplier involved for the whole application. These features all reduce integration time and cost,” said Mr. Koch.
About Gasporox
Gasporox, based in Lund in southern Sweden, develops and manufactures laser-based sensors for integration into in-line inspection and production lines for Headspace Analysis and Leak Detection. Gasporox sensors are also available in various instruments ideal for quality control and at-line inspection of packaging and support many different package formats as highly effective tools for more efficient sample testing and greener production.
The VialArch™ is a unique laser-based solution for vials to be installed on the production line for 100% quality inspection for residual oxygen and container closure integrity testing. The laser beam is passing the headspace of the moving vials to perform laser-based headspace analysis (HSA), non-destructive and with precise measurements of the residual oxygen in the headspace.
The GPX1500 Film Pharma is a compact and easy to use instrument to measure accurate the headspace of pharmaceutical bags and pouches. It’s designed to operate parameter free to have a reliable and accurate use in the pharmaceutical production and lab. The GPX1500 Vial is the Film Pharma’s sibling for rigid containers, like vials and ampoules.
The available Headspace Analysis sensors work with many types of containers like vials, ampoules, bottles, bags and trays and are available for measuring O2, CO2, H2O, or the pressure inside the inspected container and are suitable for Leak Detection on packages like trays and bags. These sensors can be integrated into production machines and also form the basis for stand-alone instruments.
Gasporox technology has been proven for performance and robustness over more than a decade in the pharmaceutical industry in the testing of parenteral drugs. It is integrated and used in machines, systems and instruments for total lab and production testing of vials and ampoules in many parenteral packaging lines.
Gasporox testing technologies are non-destructive and deterministic and recommended by <USP1207>.
Learn more at: www.gasporox.com
Resources
Click on Gasporox GPX HMI SOFTWARE for further information.