By enGenes Biotech…
Study indicates enGenes Biotech’s Shiga Toxin B subunits useful as drug carriers in cancer therapy
Vienna, Austria: – Recombinant proteins specialist CDMO enGenes Biotech GmbH (enGenes) has led a new study investigating potential oncology application of both subunit versions of its newly developed clickable Shiga Toxin B (STxB).
The study ‘Clickable Shiga Toxin B Subunit for Drug Delivery in Cancer Therapy’ has been submitted for publication by the American Chemical Society scientific journal ACS Omega.
The research team was led by senior enGenes scientist Dr Natalia Danielewicz and included two other colleagues from the Department of Biotechnology at Vienna’s University of Natural Resources and Life Sciences, Birgit Wiltschi and Gerald Striedner. They were joined by researchers from the Faculty of Biology at Freiburg University led by senior scientist Francesca Rosato and included two other colleagues Jana Tomisch and Jonas Gräber, all supervised by Winfried Römer with enGenes CEO Juergen Mairhofer as the corresponding author.
Optimised for click chemistry
Inspired by increasing focus on click chemistry receptor-mediated drug delivery in the treatment of cancer, enGenes recently presented recombinant pathogen-derived STxB subunit for use as a carrier that detects the tumour-associated glycosphingolipid globotriaosylceramide (Gb3) receptors. It is presented in two versions: STxBwt as a ‘wild-type’ or STxBAzK that incorporates noncanonical amino acid azido lysine, both manufactured using a growth-decoupled Escherichia coli (E. coli)-based expression strain.
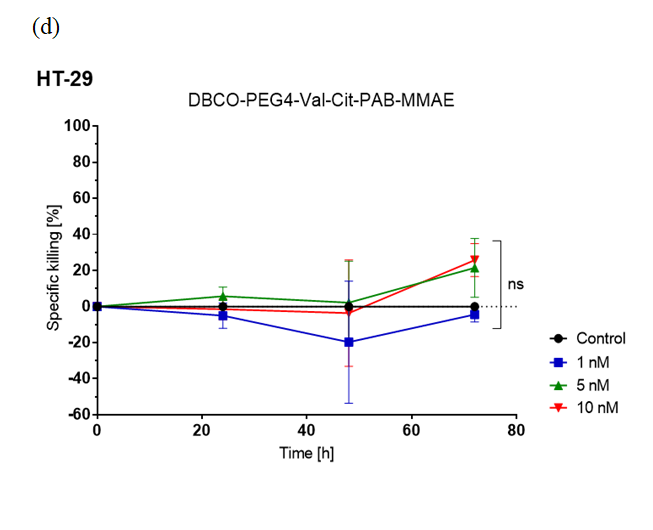
While drug conjugation via lysine or cysteine offers random drug attachment to carriers, click chemistry has the potential to improve the engineering of delivery systems as the site-specificity can eliminate interference with the active binding site of tumour ligands.
Targeting Gb3 tumour cells
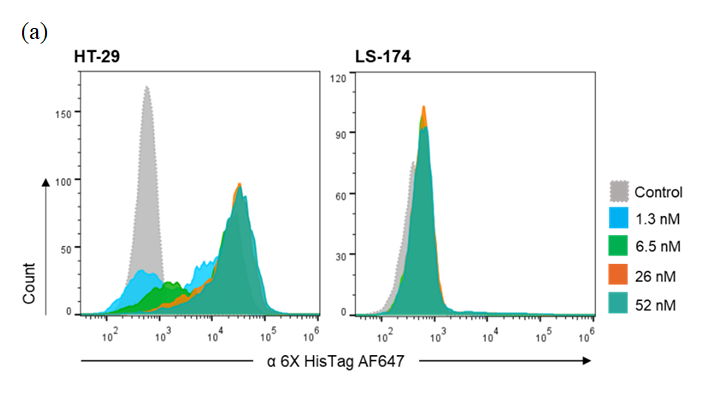
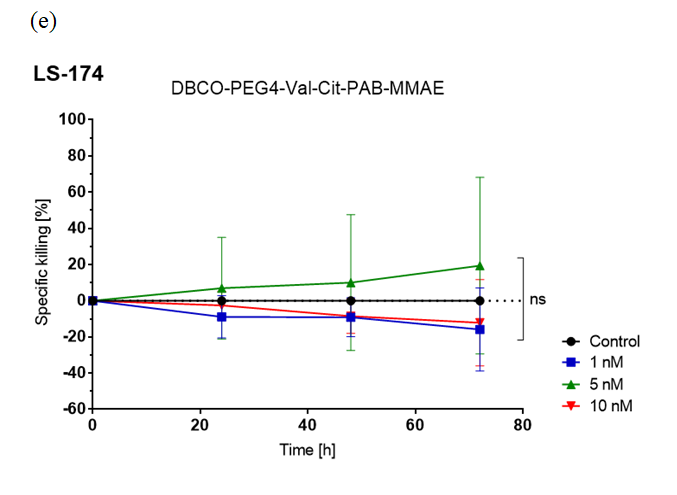
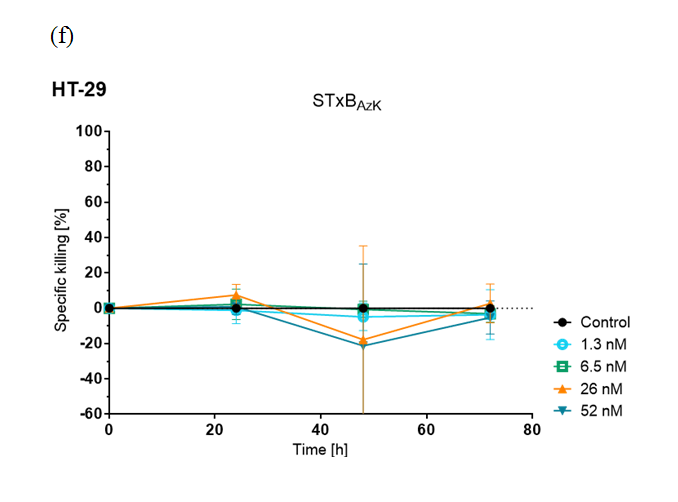
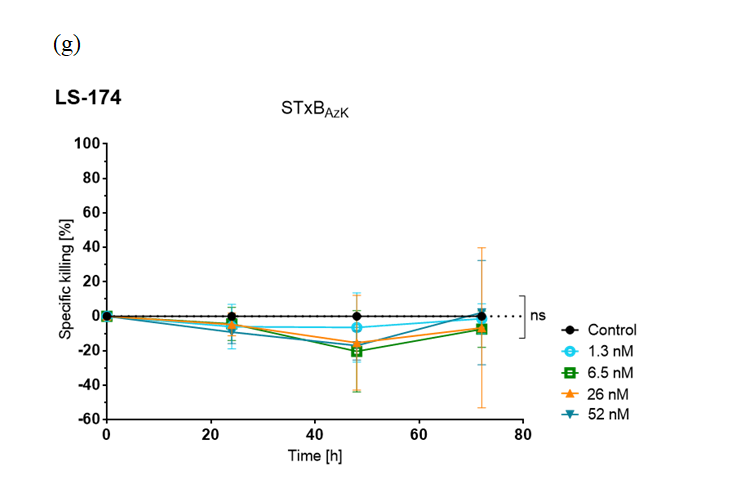
The study analyzed STxBwt and STxBAzK via flow cytometry for Gb3 receptor recognition and specificity on two human colorectal adenocarcinoma cell lines, HT-29 and LS-174, characterized by high and low Gb3 abundance, respectively.
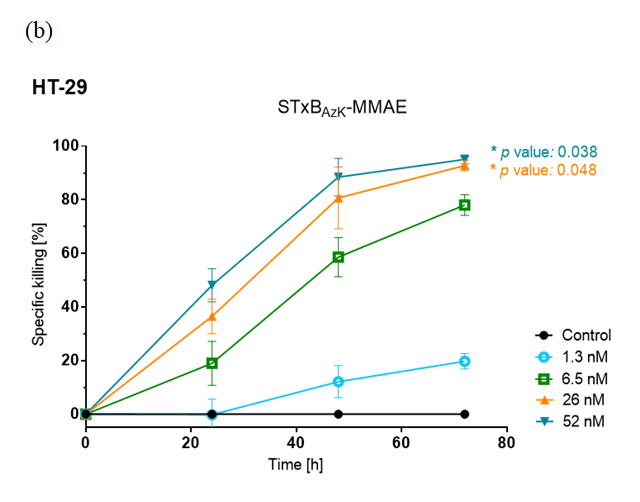
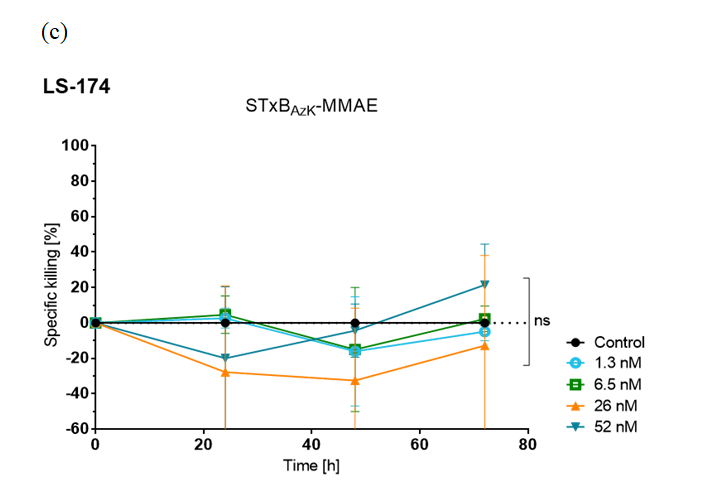
Furthermore, STxBAzK was clicked to the antineoplastic agent monomethyl auristatin E (MMAE) and evaluated in cell-killing assays for its ability to deliver the drug to Gb3-expressing tumour cells.
The research team found that the STxBAzK−MMAE conjugate induced uptake and release of the MMAE drug in Gb3-positive tumour cells, reaching 94% of HT-29 cell elimination within 72 hours of administration in low nanomolar doses while sparing LS-174 cells.
Well-functioning drug carrier
Their results indicate STxBAzK as a well-functioning drug carrier, with a possible application in cancer therapy, with the research demonstrating the feasibility of lectin carriers in delivering drugs to tumour cells, with prospects for improved cancer therapy in terms of straightforward drug attachment and effective cancer cell elimination.
In their conclusions, the authors note: “Further studies to address the stability of STxBAzK conjugates in plasma need to be conducted, to estimate the half-life of the compounds and their sensitivity to catabolism. Moreover, the exact intracellular trafficking route exploited by the carrier in target cells needs to be elucidated to gain a further understanding of the molecular mechanism of action of the STxBAzK−drug conjugate. Additionally, while the in vitro studies display encouraging properties of the STxBAzK−drug model system, in vivo mouse tumour models are required to gain further insight into the specific tumour elimination mediated by the STxBAzK carrier.”
Immunogenicity management
They suggest a range of strategies for managing STxB immunogenicity, including in silico or in vitro identification of putative B-cell and T-cell epitopes for elimination in candidate molecules, interfering with MHC II presentation/T-cell recognition or PEGylation masking.
The researchers note that other lectins of different origins, such as LecA from Pseudomonas aeruginosa or the engineered lectin Mitsuba from Mytilus galloprovincialis have been identified for their selective recognition of the Gb3 antigen, raising prospects of further advances in lectin-mediated targeted drug delivery.
Finally, they conclude: “These studies showed that the ready-to-click STxBAzK carrier offers the possibility to target glycan epitopes on tumour cells and deliver drugs effective in current cancer therapies, providing an effective appliance for targeted drug delivery in cancer research.”
About enGenes Biotech
enGenes Biotech GmbH (enGenes) is a contract research, development and manufacturing company that provides leading-edge technologies and production services focused on recombinant proteins in bacteria. The company’s mission is to provide cost-effective and scalable production of recombinant proteins at a fraction of the current cost, allied to a vision of developing a world-class portfolio of cutting-edge protein production technologies, relevant to a broad spectrum of application fields.
enGenes has developed advanced technologies to drive more cost-effective recombinant protein production processes, including its proprietary enGenes -eXpress™ E. coli platform that achieves outstanding yields of soluble and active recombinant protein. enGenes -eXpress™ has been successfully applied for the manufacturing of enzymes and biopharmaceutical products that failed to give economically feasible yields in a conventional expression host.
enGenes Biotech offers development and manufacturing services tailored to the needs of pharmaceutical and industrial biotech companies. The services include expression strain and vector development, fermentation process development and optimization, downstream process development, production of purified protein, technology transfer and scale-up support with technology out-licensing and co-development opportunities.
Resources
Click on Clickable Shiga Toxin B Subunit for Drug Delivery in Cancer Therapy to access the full study.
Click on Clickable Shiga Toxin B Subunit Supplementary Information for additional data.