By Mikron Automation
Mikron Automation leadership in automated vision inspection of medical device assembly
Boudry, Switzerland: – Technical advances by Swiss-based medical assembly process specialist Mikron form the subject of a portal article focusing on integrating automated vision led inspection into modern manufacturing processes for medical devices.
The article in GlobalData platform highlights the challenges involved in deploying advanced visual inspection technology and the opportunities it presents for manufacturing efficiency and product quality.
Vision inspection challenges
Precision is a key concern in manufacture of medical devices, where even slight variations from specification can impact product performance and jeopardize patient safety.
Advanced vision solutions use powerful analytical algorithms to offer manufacturers better quality and improved process control than ever before.
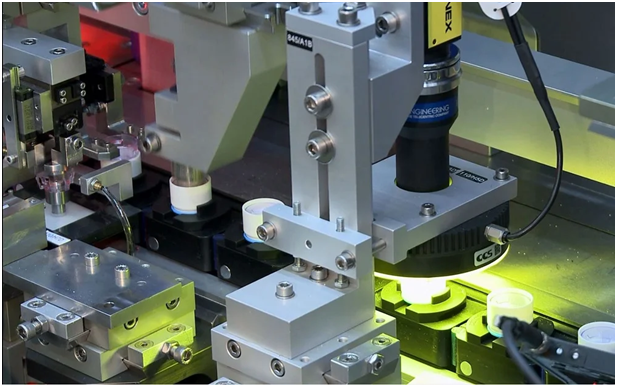
Advanced automated visual inspection technologies offer medical device manufacturers potential solutions for exercising closer control over quality and process in scale production but are challenging to implement correctly and validate. For example, image resolution is vital for algorithms to make correct accept/reject decisions. Therefore, ensuring adequate brightness and contrast in front of camera lens is a principal implementation challenge.
Opportunities
A recent Mikron Technical White Paper ‘From design to launch: the key steps to accelerating medical device development’ provides a guide to expediting medtech product launch, from design optimization to parallelizing the development of an assembly system. It sets out the scope of application possibilities and opportunities opened by video inspection as part of an overall automaton and digitization strategy.
According to GlobalData analysis suggests digitization will be a key accelerator of growth across the healthcare industry in the coming years, and companies that don’t take advantage of advanced technologies risk falling behind.
One of the most powerful applications for advanced software in manufacturing is automated quality control processes. By working with a specialized partner, medical device manufacturers can develop highly precise, specific, robust and qualified systems to guarantee their products meet quality standards.
Mikron visual inspection technologies
Mikron has been able to develop vision system that use 2D and 3D image information to determine component quality as part of a 100% automated approach. These systems combine high-resolution camera hardware with software algorithms based on a variety of criteria.
For example, high-resolution cameras can be used in flexible feeding and driving a robot to pick up a part to be fed into the machine. Similarly, vision systems can be used to automatically compute the best welding position of a 17-micron-diameter wire.
The article shows how Mikron now uses vision not just as a verifier, but as an essential element in process control, including on on-the-fly analysis at speed.
It also discusses the computational imaging that can be used to inform higher quality decisions about parts quality by taking multiple shots. Using a set of imaging techniques to combine data acquisition and processing, computational imaging enhances feature contrast, enabling the system to extract more precise information on inspected products.
Overall, this Mikron Article provides a guide to using visual inspection technologies to expedite medical device product launches, from design optimization to parallelizing the development of an assembly system.
About Mikron Automation
Mikron Automation is the leading partner for scalable and customized assembly systems – from the first idea to the highest performance solutions. Distinguished by a commitment to innovation, flexibility, unparalleled customer service, and an evolving platform portfolio, Mikron delivers state-of-the-industry solutions to the most complex assembly and testing demands.
Mikron Automation currently employs around 850 people and is headquartered in Boudry (Switzerland), a region that is regarded as the heart of the Swiss watchmaking industry. It also has sites in Denver (USA), Singapore, Shanghai (China) and Kaunas (Lithuania). The Mikron Group, a publicly-traded company with more than 100 years’ experience in precision machinery.
To date, Mikron Automation has delivered thousands of assembly solutions for a variety of drug delivery and diagnostics devices including inhalers, pens/auto-injectors, catheters, e-devices, wearables, and point-of-care tools. It also offers a constellation of high-added-value services that cover their products’ full lifecycle, wherever its customers are in the world. Mikron’s Quality Teams offer full validation support in accordance with FDA, GMP and GAMP 5 guidelines.
Further information at: www.mikron.com/automation-solutions/
Resources
Click on Looking closer: Automated vision systems for robust quality and process control to read the full GlobalData article and access the Mikron White Paper “From design to launch: the key steps to accelerating medical device development”.