By Brevetti Angela…
Brevetti Angela BFS polymer options
Brevetti Angela’s patented SYFPAC® Blow-Fill-Seal (BFS) aseptic packaging technology offers users a wide choice of different plastic polymers hat can be used to form, fill and seal purpose-made parenteral pharmaceutical containers, including syringes.
Choices over which polymer resin to use as raw material is key to ensuring final product endurance, effectiveness and stability and Brevetti Angela’s research and technical teams can provide a wealth of detailed guidance.
Plastics choices
The Brevetti Angela SYFPAC® process for combined manufacture and aseptic fill of BFS large and small bottles, vials, and ampoules is ideal for pharmaceutical aseptic filling and finishing operations, using purpose formed plastic containers. The process depends on just two supply lines: the product itself and resin granules to form the containers.
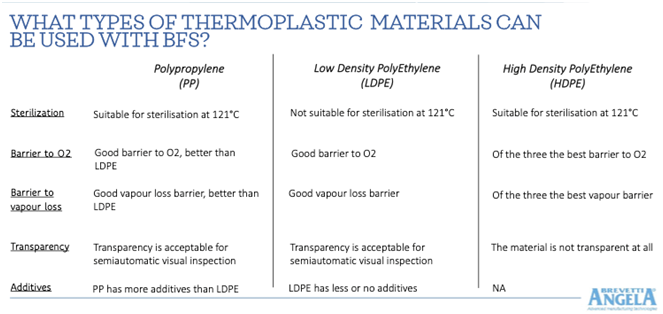
There are essentially three types of medical grade polymer that can be used in BFS blow moulding: Polypropylene (PP) and either Low-Density Polyethylene (PE or LDPE) or High-Density Polyethylene (HDPE). All three materials have different properties that make them suitable to specific applications. These properties include flexibility, transparency, barrier protection, suitability for high temperature sterilization, and purity (number of additives). These properties are summarised in the table below.
Barrier protection
The first consideration in the polymer selection process is assessment of the chemical and physical properties that impact the compatibility between resin and product, and therefore its stability. A crucial factor in product stability is insulation (barrier protection) of the liquid from environmental contaminants, micro-organisms and particulate material, as well as from light and gases.
When looking at the shelf life, barrier properties also play a key role in governing factors like the transmission rate of the moisture vapor or oxygen. Not only leachables should remain within limits during the entire shelf life, but the barrier should be valid against ingress oxygen and other gases from the environment and against loss of water or other solvent from the formulation. Product PH and concentrations must also remain within the limits over the entire envisaged shelf life.
The barrier provided by the packaging must therefore enhance product stability and shelf life by avoiding any active ingredients and/or excipients loss due to vapor diffusion or oxygen entry. In general, HDPE provides the highest barrier protection against oxygen transmission and vapor loss, with LDPE at the lower end of the scale and PP in the middle.
Physical format
However, HDPE is not a transparent material, which brings us to the second set of considerations shaping plastics choice, such as the desired appearance and form of the container, including shape, geometry, functionality, shock and crush protection and end-user requirements.
So, the second consideration is the desired mechanical characteristics of the container that include factors like rigidity, opening, dispensing, coloration and vial separation. For example, containers should be sufficiently transparent to facilitate manual or automated inspection.
In this area, PE/LDPE has some advantages over PP or HDPE in being both more flexible (‘squeezable’) as well as transparent. But this material is not compatible with high temperature sterilization at a minimum of 121°C.
Process considerations
The choice of sterilization method is highly relevant as resins exhibit different behaviours when terminally sterilized with moist heat, irradiation or other methods. One of the most common sterilization methods is at 121°C, for which PP and HDPE are both suitable. This is a key factor, since current FDA and EMA regulations stipulate that any product capable of undergoing at 121°C sterilization must now be so treated.
From the production point of view, processing characteristics of the polymer are also crucial, governing parison swell, extrusion temperature, processability and performances of extruder and knife. For example, decreasing the wall thickness of the container may result in a cost reduction, so the choice of a polymer that responds well to the BFS extrusion process can open many exciting opportunities for innovative and functionally superior packaging forms, using novel mold or extrusion forms.
BFS machinery can usually work with PP, PE or HDPE. However, while rotary machines usually work with only one specific resin per mold, SYFPAC® machines allow for a flexible choice of the polymer and instant switching of recipes in the HMI operating system settings between PP, PE or HDPE, with no need to change physical machine components.
Sustainability questions
Questions of sustainability, supply security and environmental friendliness increasingly dominate all material choices. In these fields, BFS polymers have some important advantages over traditional materials like glass. This was underlined by the recent Covid-19 pandemic, in which a worldwide shortage of glass vials became a problematic bottleneck slowing roll outs of new vaccines.
In terms of overall carbon footprint, polymers are highly competitive with glass since the manufacturing process takes fewer steps and is overall less energy and therefore emissions intensive. The BFS filling process is also less energy intensive with no need for preparatory container swashing or sterilization, for instance.
In general, both PE and PP are highly recyclable and this is also done directly within the SYFPAC system with scrap converted back into resin granules. Since the BFS containers are formed from ‘virgin’ polymers that have not been composited with other materials, these are virtually easy to recycle, although in practice most used pharma containers are normally incinerated as clinical waste, thereby generating energy instead.
Resources
Click on BFS Advantages for further explanation of the Blow-Fill-Seal process.
Click on BFS Containers to learn more.