By UGA Biopharma…
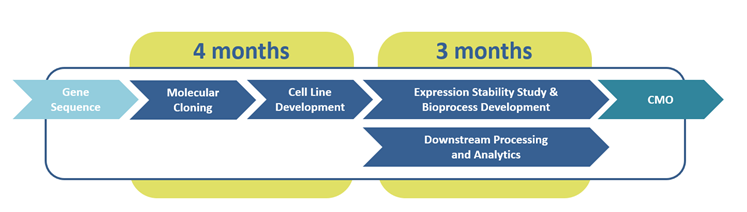
UGA Biopharma´s high-speed cell line development workflow
The UGA Biopharma GmbH high-speed workflow was established as an improved cell line development workflow that guarantees CHO cell line development on a shortened time scale.
This innovative offering is based on UGA’s well-established platform technologies including their proprietary CHO host cell lines, system-optimized expression vectors and proprietary high-performance First CHOice® media and feeds, along with a workflow methodology benefiting from experiences and capabilities that UGA Biopharma has gained over the course of many successful international development projects.
Challenges in biological drug development
Compared with conventional pharmaceuticals, biopharmaceutical development and manufacturing is considerably more complex. With living systems being involved in the manufacturing process, the quality of the desired biologic molecules depends on carefully selection and development of the cell line employed, along with a thoroughly optimized bioprocess to ensure problem-free production.
Unlike chemically produced drugs, biopharmaceuticals are highly complex molecules that are sensitive to any in process fluctuations during manufacturing or variability in performance of the production media. Furthermore, small variations in fermentation temperature, acidity, dissolved oxygen concentration and nutrient supply can affect cell clone behavior.
Optimized cell line development
UGA Biopharma has spent several years overcoming these challenges and developed a high-speed cell line development workflow. This workflow uses optimized expression vectors, in-house further refined CHO cell lines, and proprietary First CHOice® media and feeds to allow highly productive monoclonal cell lines to be developed within three to four months.
UGA Biopharma then requires just three more months for upstream processing (USP), downstream processing (DSP), and analytics (ANA) before the developed bioprocess can be transferred to a manufacturing facility. To achieve these short time scales, every work package has been optimized for shorter timelines, with packages interlaced to run concurrently wherever possible.
Higher yields
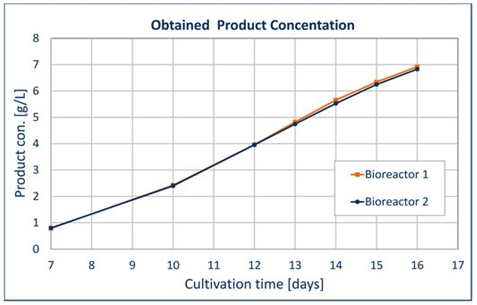
Combining this short scale workflow with UGA’s proprietary CHO host cell lines, optimized expression vectors and high-performance First CHOice® media and feeds, results in UGA being able to deliver frequently higher yields, with titers ranging from four to seven grams per liter.
UGA Biopharma guarantees consistently high quality and traceability of its cell line development activities, including a clonality report to prove monoclonality based on a whole well imaging system. In addition, UGA can also deliver royalty-free operations with CHO DG44 licenses, granted on a one-time fee.