By Tempris GmbH
Tempris GmbH
Tempris GmbH specializes in wireless and battery-free real-time product temperature measurement systems with innovative technology for monitoring product temperature and process control.
This is a unique technology developed by Tempris that enables real-time transmission of product temperature data, revolutionizing temperature control during lyophilization within the design space, ensuring high product quality and production efficiency.
This technology has the potential to revolutionize temperature-critical processes, particularly the lyophilization freeze-drying of biotechnological and pharmaceutical products. Measuring product temperature is pivotal in ensuring pharmaceutical product quality during freeze-drying.
Vision & Mission
The Tempris mission is encapsulated by its brand strapline: ‘Easify your Lyo Process’, which is a short form for automating and optimizing lyophilization process by enabling real-time monitoring of the actual products being freeze dried while they are in the chamber. This is a capability that has never previously existed.
The company’s vision is to extend this real-time data connectivity with products to extend Pharma 4.0 ‘smart factory’ functionality and digital integration into challenging pharma processes such as lyophilization.
Products & Services
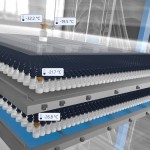
Tempris has developed sensors of unparalleled accuracy and reliability, enabling real-time monitoring in vials, dual-syringe systems, and bulk materials. Tempris wireless and battery-free product temperature sensors change the conventional narrative for process control, putting an end to the common issue known as ‘flying blind.’ The FDA’s Process Analytical Technology (PAT) initiative has introduced regulations to expedite drug approvals via in-process monitoring and streamline process transfers to new freeze-drying facilities. This transformation presents significant time and cost advantages compared to traditional approval methods, creating new prospects for the industry.
Tempris has also collaborated with partners to devise automation solutions for the computer-controlled insertion of sterile sensors. This innovation improves the efficiency and precision of sensor deployment within pharmaceutical manufacturing, resulting in a substantial 20% cost reduction through Continuous Process Verification (CPV). This automation solution also facilitates streamlined reporting, enhances product quality, bolsters patient safety expedites regulatory approvals, and accelerates transfer processes within the pharmaceutical industry. Also the automated sensor deployment ensures an aseptic process and compliance with regulations such as Annex 1.
Tempris is a Process Analytical Technology (PAT) tool that streamlines lyophilization processes by providing real-time, accurate, and reliable temperature data. One of the fundamental advantages of the technology is the same sensors can be used at every process stage of lyophilization from laboratory development through to full scale industrial process.
Tempris has developed four main product and service streams:
- Development and Manufacturing of a real-time product temperature measurement system using wireless and battery-free sensors that enable real-time product temperature measurement in the laboratory and production. Tempris quartz-based sensors are specifically designed for monitoring and optimizing freeze-drying processes and have already proven their reliability in many industrial and academic applications.
- Process Control: Application of Tempris technology as a PAT tool for monitoring product temperature and process control in freeze-drying.
- Software: Development of Tempris Lyophilization Monitoring (TLM) as a software platform that interfaces with the Tempris TIRU3 interrogation unit to record data, set all parameters, and visualize and export data.
- Implementation: Tempris supports its end users with qualified installation, system qualification, training, and technical assistance, including system maintenance and calibration.
Connected with these product focus areas are activities such as development of automated loading and placing of sensors in partnership with lyophilization and robotics partners. This innovation improves the efficiency and precision of sensor deployment within pharmaceutical manufacturing, resulting in a substantial 20% cost and time reduction through Continuous Process Verification (CPV).
Tempris works closely with leading lyophilization end-user customers, to optimize their processes for applications that can be validated by regulators. It also collaborates with universities and independent research institutions to understand the evolving needs of the industry and continuously improve its products.
Company History and Structure
Tempris was founded by Anton Mangold as iQ-mobil in 2002 to develop his invention of a wireless and battery-free sensor to measure tire pressures in Formula 1 and NASCAR motor racing. After some years Anton recognized that the same basic technology of quartz-based sensor could also be applied to temperature measurement, particularly in the extremes of lyophilization/freeze-drying.
In 2019, the company changed its name to Tempris, now marketing a third-generation sensor adapted to HCS robotic placement.
The company is headquartered in Holzkirchen, south of Munich in Bavaria, Germany. It has added distribution partners in Europe, Asia and the USA and developed collaborations with leading lyophilization specialist HOF Sonderanlagenbau GmbH, Merck International, Pfizer, and robot manufacturer Stäubli.
Quality, Regulatory and Standards
The technical core competence of Tempris lies in developing and adapting quartz-based measuring technologies for pharmaceutical applications.
The company is certified under the ISO 9001 international standard for quality management systems, as well as ISO 14001 for environmental management and sustainability.
All products are designed and engineered to comply with applicable laws and regulations, and continually improve their environmental performance, such as Good Automated Manufacturing Practice (GAMP 5) guidelines and FDA 21 CFR Part 11 / EU GMP Annex 11 for compliance of IT systems and electronic records.
Tempris sensors are specifically designed for fully automated lyophilization processes as defined by GMP Annex 1 guidelines and are constructed to cGMP standards throughout the range from laboratory scale for use in research and at pilot stage, through to production freeze-dryers.