By TAmiRNA GmbH
New study confirms useful detection limits for TAmiRNA SARS-CoV-2 rapid antibody test
Vienna, Austria: Biotech and diagnostic innovator TAmiRNA has released a new scientific study confirming that the detection limits of its IgM/IgG Antibody Rapid Test are meaningful in detecting antibody levels correlating with immunity against COVID-19 novel coronavirus.
The study results, presented in TAmiRNA’s latest Technote TN-09, shows that the rapid test was able to detect antibody concentrations down to 25 BAU/ml in serum samples, with robust detection at 50 BAU/ml. A positive antibody test thus indicates antibody levels above a proposed threshold for protective immunity against SARS-CoV-2.
TAmiRNA Sars-CoV-2 IgM/IgG Rapid test
The TAmiRNA Sars-CoV-2 IgM/IgG Antibody Rapid test enables qualitative detection of IgG and IgM antibodies directed against the SARS-CoV-2 spike protein in under 15 minutes from whole blood or serum samples .
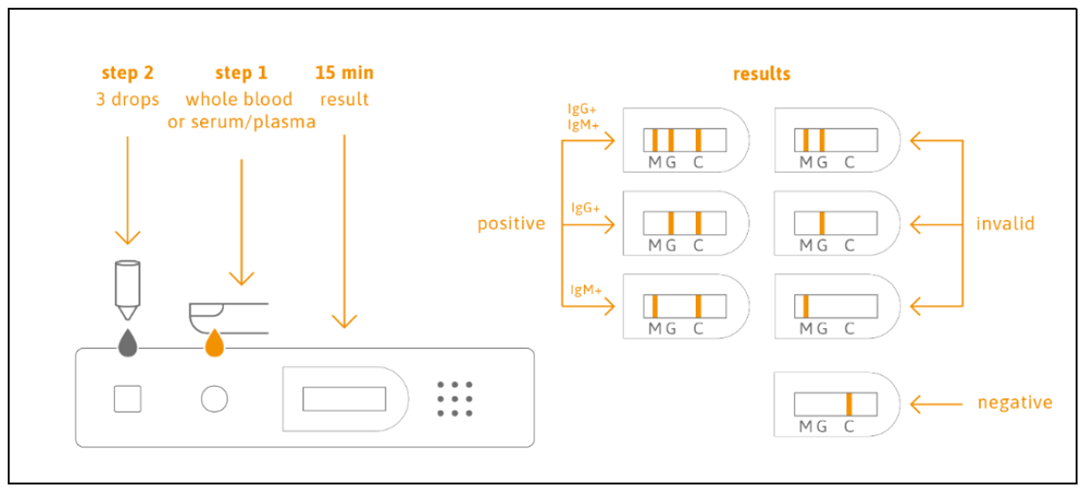
The Rapid Test is based on the principle of immunochromatographic detection of SARS-CoV-2-IgG/IgM antibodies with specificity against the S1 spike protein. A drop of whole blood derived from a finger stick is dropped into the sample window and absorbed into the cassette by capillary action , where it mixes with the SARS-CoV-2 antigen dye conjugate and flows through the pre-coated membrane (Figure 1).
An independent validation study at Medical University of Innsbruck based on 350 patient samples demonstrated sensitivity of 98.25% with 100% specificity, demonstrating a diagnostic accuracy comparable to ELISA tests, the current gold standard, while being able to be performed on-site without laboratory equipment. In addition to the detection of previous infections, the test can also be used to confirm the antibody-based immune response of individuals to vector- as well as mRNA vaccines2.
Informative value
The detection of neutralizing antibodies against the receptor-binding domain of the SARS-CoV-2 virus has become increasingly important throughout the ongoing COVID-19 pandemic and will become even more relevant in the future to assess ‘herd immunity’ of the population through exposure to the virus or through vaccination, in order to predict how the disease might affect us in the future, and plan appropriate countermeasures.
Other scenarios in which TAmiRNA’s antibody test can aid decision making include:
- To detect a previous infection with the SARS-CoV-2 virus when no viral testing was carried out at the time of infection
- To detect a symptomless previous infection
- To aid diagnosis and therapeutic decisions when a person develops symptoms consistent with COVID-19, but the viral test is negative
- To verify the response to a vaccine
- To enable participation in research studies aiming at understanding the short- and long-term effects of SARS-CoV-2 infections on human health.
Study Aims and Method
The study aimed to define the test’s limit of detection and its meaningfulness against current and proposed thresholds for with immunity against SARS-CoV-2. The study team prepared a dilution series using the First WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin (NIBSC Code: 20/136), which contains the freeze-dried equivalent of 0.25 ml of pooled plasma obtained from eleven individuals recovered from COVID19 and has a concentration of 1000 Binding Antibody Units (BAUs) per milliliter, corresponding to 1000 International Units (IUs) per ml after reconstitution. The team then analyzed this dilution series using the rapid test, comparing established limits of detection against other tests to assess meaningfulness of the results.
Methodology
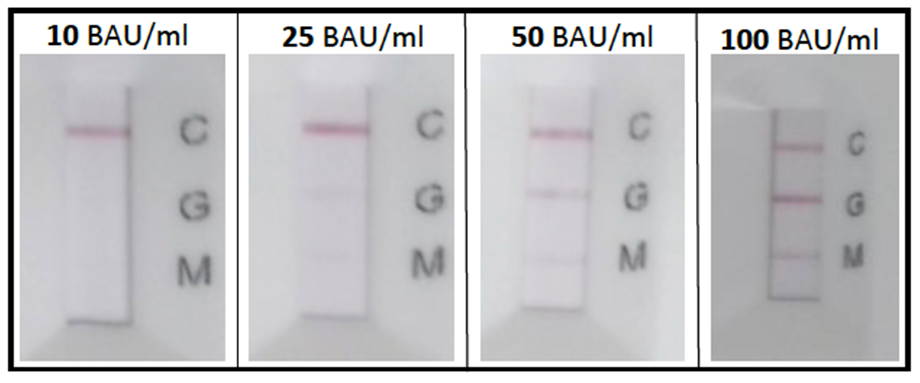
The standard was then diluted in tested negative serum (COVID-19 Remnant Negative Serum Samples, provided by Precision for Medicine, Lot: 29653) to get the following concentrations: 100, 50, 25, 10, 5 and 1 BAU/ml. 10μl of the standards were transferred into the sample window of the test cassette. Afterwards, 120μL (3 drops) sample buffer was applied in the buffer window. Tests were conducted as duplicates. After 15 minutes, results were read, photographed and documented. Only valid test results (with visible control band) were considered for the evaluation. IgM and IgG bands were classified as negative, weak-positive, or positive.
Results
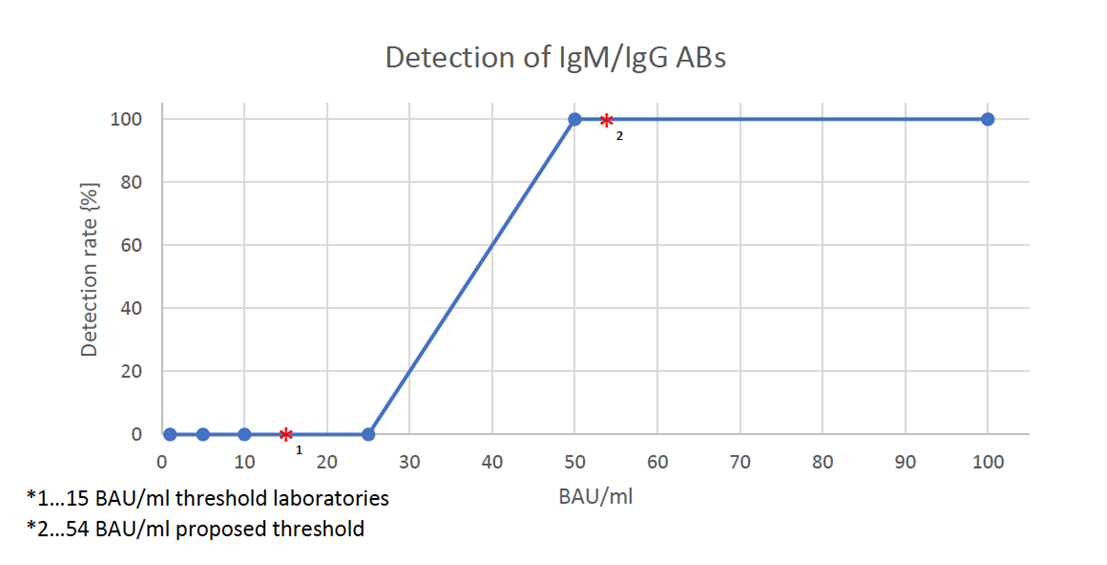
The dilution series with the NIBSC standard in negative serum resulted in a detection limit between 25 and 50 binding antibody units (BAU) per ml. Values above 15 BAU/ml have been correlated to the presence of neutralizing antibodies, thus a positive rapid test result indicates the presence of sufficient neutralizing antibodies1.
The rapid tests were able to detect concentrations down to 25 BAU/ml in negative serum samples. However, the 25 BAU/ml marks were already faint, thus the limit of detection is seen above 25 BAU/ml. (Figure 2).
Relevance of the limit of detection
Diagnostic laboratories in Austria have defined thresholds of IgM/IgG antibodies that indicate immunity for Sars-CoV-2. Generally, a titer of above 0.8 BAU/ml indicates presence of antibodies; however, 15 BAU/ml is considered the minimum concentration at which protection from infection can be assumed . A recent study by Khoury et al. (2021) estimated protective quantity of neutralizing ABs for SARS-CoV-2 above 54 IU/ml.3
Since the limit of detection of TAmiRNA’s antibody test is above 15 BAU/ml, and robust detection with strong, clear bands was observed at 50 BAU/ml, a positive test result indicates assumed immunity against COVID-19.
References:
- Values are adopted from Labros.at (https://www.labors.at/sars-cov-2-antikoerper-testverfahren-2/)
- TAmiRNA TechNote TN-07 published March 2021: https://www.tamirna.com/wp-content/uploads/2021/04/TN07-technote_internal-anibody-study_v2_DE.pdf
- Khoury, D.S., Cromer, D., Reynaldi, A. et al. , 2001, Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27, 1205–1211 (2021). https://doi.org/10.1038/s41591-021-01377-8
About TAmiRNA
TAmiRNA specializes in technologies for profiling levels of blood-circulating miRNAs and developing multi-parametric classification algorithms (“signatures”). TAmiRNA uses these technologies to develop minimal-invasive diagnostic tests for drug development, early diagnosis and prognosis of disease, and as companion diagnostic tests to support treatment decisions.
For its work on circulating microRNAs in bone disease, TAmiRNA is receiving funding from the European Union’s Horizon 2020 research and innovation program under the MARIE SKŁODOWSKA-CURIE grant agreement no. 860898.
More information available at: www.tamirna.com
Resources
Click on TAmiRNA Technote 09 to access full study.
Click on TAmiRNA Technote 07 for Verification of immune response after SARS-CoV-2 vaccination using TAmiRNA SARS-CoV-2 IgM/IgG Antibody Rapid Test.
.