By CERBIOS-PHARMA SA
Cerbios nears completion of new cGMP cytotoxic unit for increased HPAPIs and Payloads production
Lugano, Switzerland: – Active Pharmaceutical Ingredient specialist Cerbios-Pharma SA (Cerbios) says it has almost completed building of its new manufacturing facility dedicated to Cytotoxic molecules.
The new building at Lugano site will house two additional cGMP production lines along with additional space for dedicated R&D and QC Labs.
GMP manufacturing
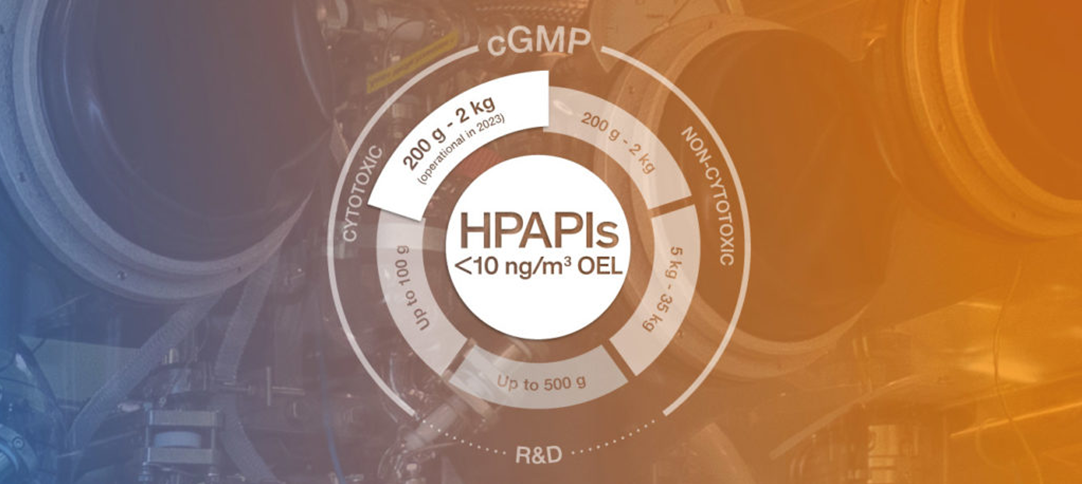
The construction of the new building is almost completed and the next 10-12 months will be dedicated to the equipment installation and the plant approval. The new cGMP unit (Category 4 SafeBridge®) will become operational in Q2 2023 and will be suitable for hosting production ranging from 200mg to 2kgs per batch.
Cerbios has been an active HPAPI developer and producer since 1993, with ability to handle high containment production of drug substances up to Category 4 SafeBridge® standards (OEL<10 ng/m3). With dedicated R&D and scale up facilities and cGMP manufacturing lines ranging from gram scale up to 30+ Kg batch sizes (Category 3 SafeBridge®), Cerbios manufactures both Cytotoxic and non-Cytotoxic molecules.
Boosted CDMO service portfolio
Cerbios-Pharma SA Chief Commercial Officer Denis Angioletti commented: “The investment has the purpose of implementing up to date technologies and increasing the number of manufacturing lines, designed on the basis of modern concepts that ensure we can meet the latest safety standards while retaining maximum flexibility, resulting in an additional tool to our capabilities.”
Along with the conjugation suites for Antibody Drug Conjugates (ADCs), this new production line completes the Cerbios CDMO chemical services portfolio and makes it an ideal partner for HPAPIs, Payloads and ADCs, with ability to cover all development and manufacturing phases, from production of non-cGMP batches for preclinical tests, through to clinical to commercial supply.
About Cerbios-Pharma
Cerbios is a privately held company located in Lugano, Switzerland, specialized in manufacturing Pharma Products and in offering CDMO services.
Cerbios is the ideal CDMO partner from clinical to commercial supply of API, HPAPIs, Payloads, ADCs, Proteins and Antibodies. Services offered include Process & Analytical Development, Scale-up and Clinical Supply, Industrial Validation, Commercial Supply, Full CMC Regulatory Support. Proveo (TM) is a Cerbios division recently created to provide end-to-end development and manufacturing services for ADCs.
Cerbios is also a global leading supplier of a portfolio of Generic APIs primarily used in Oncology and for the treatment of Respiratory and Dermatological disorders. Cerbios has additionally a 40 years’ experience in the probiotics field: the portfolio includes a FDF based on proprietary strain SF68® for treatment of diarrhea and dysbiosis by antibiotics and two new Food Supplements for the management of ACNE & Seborrheic dermatitis and to boost immune response.
Learn more at: https://cerbios.swiss
Resources
Click on Cerbios announces the near finalization of a new cGMP unit for cytotoxic to see original post.
Click on Cerbios-Pharma ADC Services for detailed information.