By TAmiRNA GmbH
TAmiRNA toxomiR™ microRNA panel for toxicology testing
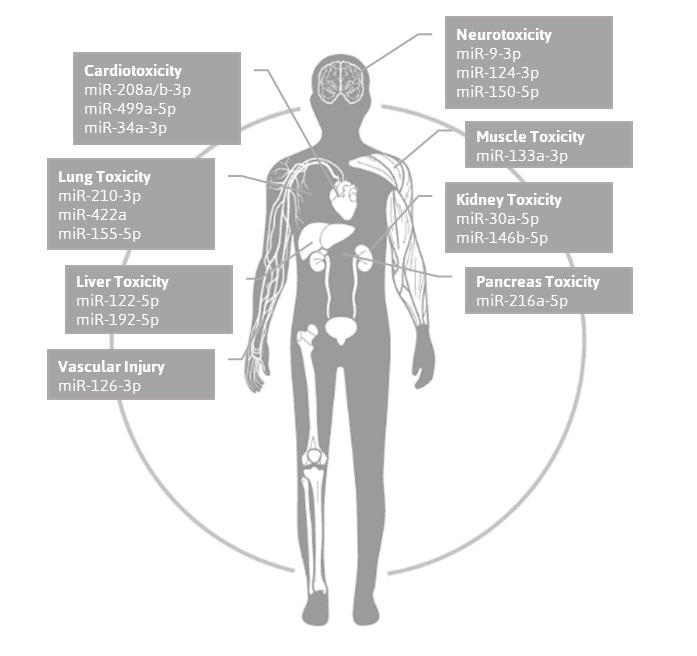
MicroRNAs are early indicators and useful tools to detect drug-induced toxicity. TAmiRNA’s new toxomiRTM testing service allows clients to ship samples (serum or plasma) to the TAmiRNA service lab for toxicology analysis using its carefully designed and qualified toxomiR microRNA panel. The panel consists of 24 toxicology biomarkers and assay controls that enable parallel, non-invasive monitoring of toxic effects on eight organs and detection of endocrine disrupting activity.
The service can accept unlimited numbers of samples and delivers results with data normalization, statistical analysis and results report and full documentation.
Applications
The toxomiR™ microRNA panel is based on the repeated observations by various scientific groups that toxicant-induced changes in the transcription and secretion of toxomiRs are highly sensitive to toxic effects occurring in the liver, kidney, brain, heart, muscle, lung, pancreas, vascular injury, and due to endocrine disruption.
TAmiRNA can now analyze toxomiRs in serum or plasma as an in-house service that will allow clients from academia and industry to gain a fast and uncomplicated insight into the nature of their samples in the context of toxic injury in different organs.
The service is highly applicable to academic research and clinical research groups and pharmaceutical companies who are active in drug development and toxicology research all over the world.
It allows (I) non-invasive screening of toxic effects using serum or plasma, (II) identifying and monitoring safety risks in drug development and (III) early detection of tissue injury.
It also is highly useful for clients lacking the required laboratory infrastructure to undertake toxicology testing or who demand high-throughput capacity and very fast turnaround.
Features
The toxomiRTM service is intended for translational and clinical research as a support for bulk toxicity testing of multiple samples and tissues.
It allows client organizations to take advantage of TAmiRNA´s highly capable and advanced laboratory environment to achieve highest data quality.
There are no restrictions on sample numbers that can be accepted for batch testing using the toxomiR panel. Furthermore, each analysis requires only 200µL per serum or plasma sample.
Data analysis is based on TAmiRNA´s data normalization pipeline, which ensures excellent data comparability, even across multiple centers.
The service includes quality control over all samples via data normalization and full analysis, with formal results reporting.
Benefits
The toxomiR service allows academic and clinical research groups to obtain fast and uncomplicated insights into the nature of their samples.
The advanced technology allows very rapid turnaround times to be achieved. Staff who are expert in RNA isolation conduct all sample handling and testing, ensuring excellent data quality.
Clients receive high quality data, ready for presentation, publication and further analysis.
Resources
Click on toxomiR testing for detailed information.
Click on microRNAs for detail on TAmiRNA’s microRNA technologies.
Click on biomarkers for detail on TAmiRNA’s use of biomarkers.